Abstract
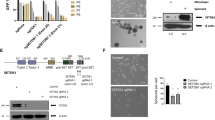
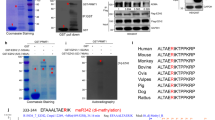
Enhancer of zeste homologue 2 (EZH2) is the catalytic subunit of Polycomb repressive complex 2 (PRC2) and catalyses the trimethylation of histone H3 on Lys 27 (H3K27), which represses gene transcription. EZH2 enhances cancer-cell invasiveness and regulates stem cell differentiation. Here, we demonstrate that EZH2 can be phosphorylated at Thr 487 through activation of cyclin-dependent kinase 1 (CDK1). The phosphorylation of EZH2 at Thr 487 disrupted EZH2 binding with the other PRC2 components SUZ12 and EED, and thereby inhibited EZH2 methyltransferase activity, resulting in inhibition of cancer-cell invasion. In human mesenchymal stem cells, activation of CDK1 promoted mesenchymal stem cell differentiation into osteoblasts through phosphorylation of EZH2 at Thr 487. These findings define a signalling link between CDK1 and EZH2 that may have an important role in diverse biological processes, including cancer-cell invasion and osteogenic differentiation of mesenchymal stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cao, R. et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039–1043 (2002).
Kuzmichev, A., Nishioka, K., Erdjument-Bromage, H., Tempst, P., Reinberg, D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 16, 2893–2905 (2002).
Cao, R., Zhang, Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr. Opin. Genet. Dev. 14, 155–164 (2004).
Varambally, S. et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419, 624–629 (2002).
Kleer, C. G. et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc. Natl Acad. Sci. USA 100, 11606–11611 (2003).
Bracken, A. P. et al. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 22, 5323–5335 (2003).
Bachmann, I. M. et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J. Clin. Oncol. 24, 268–273 (2006).
Gonzalez, M. E. et al. Downregulation of EZH2 decreases growth of estrogen receptor-negative invasive breast carcinoma and requires BRCA1. Oncogene 28, 843–853 (2009).
Van Hoof, D. et al. Unraveling the human embryonic stem cell phosphoproteome. Cell Stem Cell 5, 214–226 (2009).
Imbach, P. et al. 2, 6, 9-trisubstituted purines: optimization towards highly potent and selective CDK1 inhibitors. Bioorg. Med. Chem. Lett. 9, 91–96 (1999).
van den Heuvel, S. and Harlow, E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262, 2050–2054 (1993).
Bracken, A. P. et al. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 20, 1123–1136 (2006).
Boyer, L. A. et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441, 349–353 (2006).
Lee, T. I. et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125, 301–313 (2006).
Barry, F. P. & Murphy, J. M. Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 36, 568–584 (2004).
Bruder, S. P. et al. Mesenchymal stem cells in bone development, bone repair and skeletal regeneration therapy. J. Cell Biochem. 56, 283–294 (1994).
Mackay, A. M. et al. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 4, 415–428 (1998).
Pittenger, M. F. et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 (1999).
Jaiswal, N., Haynesworth, SE., Caplan, A. I. and Bruder, S. P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 64, 295–312 (1997).
Chen, S. et al. Cyclin-dependent kinases regulate epigenetic gene silencing through phosphorylation of EZH2. Nat. Cell Biol. 12, 1108–1114 (2010).
Moffat, J. et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 (2006).
Lee, D. F. et al. IKKβ suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell 130, 440–455 (2007).
Wang, H. et al. Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science 293, 853–857 (2001).
Acknowledgements
We thank S. A. Miller and J. Hsu for editorial assistance. We thank A. M. Labaff for characterization of EED antibody. This work was supported by grants from National Institutes of Health (grant R01 CA109311), Kadoorie Charitable Foundations, National Breast Cancer Foundation Inc. and the M. D. Anderson Cancer Center/China Medical University and Hospital Sister Foundation Funds (to M.-C.H), NSC 96-3111-B-039 and NSC 97-3111-B-039 (to M.-C.H., L.-Y.L. and S.-P.Y), NHRI-EX98-9603BC, DOH97-TD-I-111-TM003, DOH98-TD-I-111-TM002 (to L.-Y.L.), DOH97-TD-G-111-041 (to M.-C.H.), DOH99-TD-C-111-005, NSC99-2632-B-039-001-MY3 (to L. –Y.L. and M. –C.H.). In memory of Mrs Serena Lin-Guo for her courageous battle against breast cancer.
Author information
Authors and Affiliations
Contributions
M.-C.H., Y.W. and L.-Y.L. designed the project and wrote the paper. M.-C.H. and L.-Y.L. supervised the research. Y.W. performed most of the experiments in Figs 1, 2, 3. Y.-H.C. performed mesenchymal stem cell experiments in Figs 4 and 5 and experiments investigating the interaction between EZH2 and CDK1. C.-C.L. performed the mass spectrometry analysis. C.-Y.L. generated and characterized the phospho-EZH2 antibody. S.-P.Y. collected and characterized primary human mesenchymal stem cells. J. L., B.S., C. -C.Y. and J.-Y.Y. assisted with experiments. All authors participated in interpreting the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1595 kb)
Supplementary Table 1
Supplementary Information (XLS 531 kb)
Supplementary Table 2
Supplementary Information (XLS 27 kb)
Supplementary Table 3
Supplementary Information (PDF 145 kb)
Supplementary Table 4
Supplementary Information (PDF 114 kb)
Rights and permissions
About this article
Cite this article
Wei, Y., Chen, YH., Li, LY. et al. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat Cell Biol 13, 87–94 (2011). https://doi.org/10.1038/ncb2139
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2139
This article is cited by
-
FLT3 tyrosine kinase inhibition modulates PRC2 and promotes differentiation in acute myeloid leukemia
Leukemia (2024)
-
Phosphorylation of EZH2 differs HER2-positive breast cancer invasiveness in a site-specific manner
BMC Cancer (2023)
-
Breast cancer patient-derived microtumors resemble tumor heterogeneity and enable protein-based stratification and functional validation of individualized drug treatment
Journal of Experimental & Clinical Cancer Research (2023)
-
EZH2 K63-polyubiquitination affecting migration in extranodal natural killer/T-cell lymphoma
Clinical Epigenetics (2023)
-
Dysregulation of histone modifications in bone marrow mesenchymal stem cells during skeletal ageing: roles and therapeutic prospects
Stem Cell Research & Therapy (2023)