Abstract
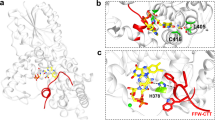
Circadian rhythms influence many behaviours and diseases1,2. They arise from oscillations in gene expression caused by repressor proteins that directly inhibit transcription of their own genes. The fly circadian clock offers a valuable model for studying these processes, wherein Timeless (Tim) plays a critical role in mediating nuclear entry of the transcriptional repressor Period (Per) and the photoreceptor Cryptochrome (Cry) entrains the clock by triggering Tim degradation in light2,3. Here, through cryogenic electron microscopy of the Cry–Tim complex, we show how a light-sensing cryptochrome recognizes its target. Cry engages a continuous core of amino-terminal Tim armadillo repeats, resembling how photolyases recognize damaged DNA, and binds a C-terminal Tim helix, reminiscent of the interactions between light-insensitive cryptochromes and their partners in mammals. The structure highlights how the Cry flavin cofactor undergoes conformational changes that couple to large-scale rearrangements at the molecular interface, and how a phosphorylated segment in Tim may impact clock period by regulating the binding of Importin-α and the nuclear import of Tim–Per4,5. Moreover, the structure reveals that the N terminus of Tim inserts into the restructured Cry pocket to replace the autoinhibitory C-terminal tail released by light, thereby providing a possible explanation for how the long–short Tim polymorphism adapts flies to different climates6,7.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The cryo-EM density maps and the atomic model for Tim–Cry have been deposited in the Electron Microscopy Data Bank (EMD-27335) and the PDB (8DD7), respectively. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium through the PRIDE partner repository with the dataset identifier PXD034054. All other data are contained within the Supplementary Information. Reagents are available from the authors upon request.
Code availability
No custom computer code was used in the study.
References
Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 (2017).
Crane, B. R. & Young, M. W. Interactive features of proteins composing eukaryotic circadian clocks. Ann. Rev. Biochem. 83, 191–219 (2014).
Foley, L. E. & Emery, P. Drosophila cryptochrome: variations in blue. J. Biol. Rhythms 35, 16–27 (2020).
Top, D., Harms, E., Syed, S., Adams, E. L. & Saez, L. GSK-3 and CK2 kinases converge on Timeless to regulate the master clock. Cell Rep. 16, 357–367 (2016).
Jang, A. R., Moravcevic, K., Saez, L., Young, M. W. & Sehgal, A. Drosophila TIM binds Importin α1, and acts as an adapter to transport PER to the nucleus. PLOS Genet. 11 e004974 (2015).
Tauber, E. et al. Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science 316, 1895–1898 (2007).
Deppisch, P. et al. Adaptation of Drosophila melanogaster to long photoperiods of high-latitude summers is facilitated by the ls-timeless allele. J. Biol. Rhythms 37, 185–201 (2022).
Lin, F. J., Song, W., Meyer-Bernstein, E., Naidoo, N. & Sehgal, A. Photic signaling by cryptochrome in the Drosophila circadian system. Mol. Cell. Biol. 21, 7287–7294 (2001).
Chaves, I. et al. The cryptochromes: blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 62, 335–364 (2011).
Ozturk, N. Phylogenetic and functional classification of the photolyase/cryptochrome family. Photochem. Photobiol. 93, 104–111 (2017).
Parico, G. C. G. et al. The human CRY1 tail controls circadian timing by regulating its association with CLOCK:BMAL1. Proc. Natl Acad. Sci. USA 117, 27971–27979 (2020).
Berndt, A. et al. A novel photoreaction mechanism for the circadian blue light photoreceptor Drosophila cryptochrome. J. Biol. Chem. 282, 13011–13021 (2007).
Vaidya, A. T. et al. Flavin reduction activates Drosophila cryptochrome. Proc. Natl Acad. Sci. USA 110, 20455–20460 (2013).
Ozturk, N., Selby, C. P., Annayev, Y., Zhong, D. & Sancar, A. Reaction mechanism of Drosophila cryptochrome. Proc. Natl Acad. Sci. USA 108, 516–521 (2011).
Lin, C. F., Top, D., Manahan, C. C., Young, M. W. & Crane, B. R. Circadian clock activity of cryptochrome relies on tryptophan-mediated photoreduction. Proc. Natl Acad. Sci. USA 115, 3822–3827 (2018).
Lin, C., Schneps, C. M., Chandrasekaran, S., Ganguly, A. & Crane, B. R. Mechanistic insight into light-dependent recognition of Timeless by Drosophila Cryptochrome. Structure 30, 851–861 (2022).
Chandrasekaran, S. et al. Tuning flavin environment to detect and control light-induced conformational switching in Drosophila cryptochrome. Commun. Biol. 4, 249 (2021).
Ganguly, A. et al. Changes in active site histidine hydrogen bonding trigger cryptochrome activation. Proc. Natl Acad. Sci. USA 113, 10073–10078 (2016).
Koh, K., Zheng, X. Z. & Sehgal, A. JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science 312, 1809–1812 (2006).
Peschel, N., Chen, K. F., Szabo, G. & Stanewsky, R. Light-dependent interactions between the Drosophila circadian clock factors Cryptochrome, Jetlag, and Timeless. Curr. Biol. 19, 241–247 (2009).
Yu, D. et al. Optogenetic activation of intracellular antibodies for direct modulation of endogenous proteins. Nat. Methods 16, 1095–1100 (2019).
Gil, A. A. et al. Optogenetic control of protein binding using light-switchable nanobodies. Nat. Commun. 11, 4044 (2020).
Busza, A., Emery-Le, M., Rosbash, M. & Emery, P. Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science 304, 1503–1506 (2004).
Dissel, S. et al. A constitutively active cryptochrome in Drosophila melanogaster. Nat. Neurosci. 7, 834–840 (2004).
Hemsley, M. J. et al. Linear motifs in the C-terminus of D-melanogaster cryptochrome. Biochem. Biophys. Res. Commun. 355, 531–537 (2007).
Vodovar, N., Clayton, J. D., Costa, R., Odell, M. & Kyriacou, C. P. The Drosophila clock protein Timeless is a member of the Arm/HEAT family. Curr. Biol. 12, R610–R611 (2002).
Holzer, S. et al. Crystal structure of the N-terminal domain of human Timeless and its interaction with Tipin. Nucleic Acids Res. 45, 5555–5563 (2017).
Zoltowski, B. D. et al. Structure of full-length Drosophila cryptochrome. Nature 480, 396–399 (2011).
Levy, C. et al. Updated structure of Drosophila cryptochrome. Nature 495, E3–E4 (2013).
Czarna, A. et al. Structures of Drosophila cryptochrome and mouse cryptochrome1 provide insight into circadian function. Cell 153, 1394–1405 (2013).
Sandrelli, F. et al. A molecular basis for natural selection at the timeless locus in Drosophila melanogaster. Science 316, 1898–1900 (2007).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Kurien, P. et al. TIMELESS mutation alters phase responsiveness and causes advanced sleep phase. Proc. Natl Acad. Sci. USA 116, 12045–12053 (2019).
Schmalen, I. et al. Interaction of circadian clock proteins CRY1 and PER2 is modulated by zinc binding and disulfide bond formation. Cell 157, 1203–1215 (2014).
Fribourgh, J. L. et al. Dynamics at the serine loop underlie differential affinity of cryptochromes for CLOCK:BMAL1 to control circadian timing. Elife 9, e55275 (2020).
Nangle, S. N. et al. Molecular assembly of the Period-Cryptochrome circadian transcriptional repressor complex. Elife 3, e03674 (2014).
Engelen, E. et al. Mammalian TIMELESS is involved in period determination and DNA damage-dependent phase advancing of the circadian clock. PLoS ONE 8, e56623 (2013).
Baretic, D. et al. Cryo-EM structure of the fork protection complex bound to CMG at a replication fork. Mol. Cell 78, 926–940 (2020).
Xie, S. et al. Timeless interacts with PARP-1 to promote homologous recombination repair. Mol. Cell 60, 163–176 (2015).
Stanewsky, R. et al. The cry(b) mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95, 681–692 (1998).
Wang, Y. J., Veglia, G., Zhong, D. P. & Gao, J. L. Activation mechanism of Drosophila cryptochrome through an allosteric switch. Sci. Adv. 7, eabg3815 (2021).
Berntsson, O. et al. Photoactivation of Drosophila melanogaster cryptochrome through sequential conformational transitions. Sci. Adv. 5, eaaw1531 (2019).
Glas, A. F., Schneider, S., Maul, M. J., Hennecke, U. & Carell, T. Crystal structure of the T(6-4)C lesion in complex with a (6-4) DNA photolyase and repair of UV-induced (6-4) and Dewar photolesions. Chemistry 15, 10387–10396 (2009).
Nangle, S., Xing, W. M. & Zheng, N. Crystal structure of mammalian cryptochrome in complex with a small molecule competitor of its ubiquitin ligase. Cell Res. 23, 1417–1419 (2013).
Rosensweig, C. et al. An evolutionary hotspot defines functional differences between CRYPTOCHROMES. Nat. Commun. 9, 1138 (2018).
Tewari, R., Bailes, E., Bunting, K. A. & Coates, J. C. Armadillo-repeat protein functions: questions for little creatures. Trends Cell Biol. 20, 470–481 (2010).
Choi, H. J. et al. A conserved phosphorylation switch controls the interaction between cadherin and β-catenin in vitro and in vivo. Dev. Cell 33, 82–93 (2015).
Goldfarb, D. S., Corbett, A. H., Mason, D. A., Harreman, M. T. & Adam, S. A. Importin α: a multipurpose nuclear-transport receptor. Trends Cell Biol. 14, 505–514 (2004).
Catimel, B. et al. Biophysical characterization of interactions involving importin-α during nuclear import. J. Biol. Chem. 276, 34189–34198 (2001).
Harreman, M. T., Hodel, M. R., Fanara, P., Hodel, A. E. & Corbett, A. H. The auto-inhibitory function of importin α is essential in vivo. J. Biol. Chem. 278, 5854–5863 (2003).
Muok, A. R. et al. Engineered chemotaxis core signaling units indicate a constrained kinase-off state. Sci. Signal. 13, eabc1328 (2020).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D 74, 519–530 (2018).
Morin, A. et al. Collaboration gets the most out of software. Elife 2, e01456 (2013).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D 75, 861–877 (2019).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Pintilie, G. et al. Measurement of atom resolvability in cryo-EM maps with Q-scores. Nat. Methods 17, 328–334 (2020).
Kallberg, M. et al. Template-based protein structure modeling using the RaptorX web server. Nat. Protoc. 7, 1511–1522 (2012).
Holm, L. & Laakso, L. M. Dali server update. Nucleic Acids Res. 44, W351–W355 (2016).
Acknowledgements
We thank Q. Fu and S. Zhang of the Proteomics and Metabolomics Facility of Cornell University for providing the mass spectrometry data; K. Spoth and M. Silvestry-Ramos for help with electron microscopy instrumentation; Y. Li for suggesting use of the ALFA tag and experimental assistance; G. Merz, T.-T. Nguyen and C. Aplin for general aid; and R. Cerione and M. Young for helpful feedback. Figure 1a, the inset in Fig. 2b, Fig. 4d and Supplementary Fig. 1 were created with BioRender.com. This work was supported by NIH grants R35GM122535 to B.R.C. and 1S10 OD017992-01 for the Orbitrap Fusion mass spectrometer. This work made use of the Cornell Center for Materials Research Shared Facilities that are supported through the NSF (DMR-1719875).
Author information
Authors and Affiliations
Contributions
All authors contributed to each aspect of the study, but primarily, C.L. and C.C.D. developed the SWFTI assay; C.L. carried out Cry–Tim binding assays; C.L. assisted by C.C.D. developed methods to produce the Cry–Tim complex; C.L. prepared the complex for structural analysis; S.F. prepared samples for cryo-EM, and collected and processed cryo-EM data; S.F. and B.R.C. built the Cry–Tim model; C.L., S.F. and B.R.C. analysed the structure; C.L., S.F., C.C.D. and B.R.C. wrote the manuscript; B.R.C. conceived of and oversaw the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Amita Sehgal, Sebastian Westenhoff and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Cry-Tim purification and cryo-EM workflow.
Purification of Cry-Tim complexes for cryo-EM. (a) SDS-PAGE gel showing complex after elution from the ALFA resin, followed by crosslinking, followed by SEC. (b) SEC trace of purified complex, peak I: aggregates, peak II: Cry-Tim complex, and peak III: excessive ALFA peptide. (c) Processing workflow for Cry-Tim collected on a Talos Arctica (Thermo Fischer Scientific) operated at 200 keV using a Gatan K3 direct electron camera. The red box indicates the final reconstruction in this study. Data processing was carried out in CryoSparc and RELION. Motion Correction was performed by RELION (MotionCor2); with Contrast Transfer Function (CTF) applied in CryoSparc. Topaz was used for particle classification. Overall resolution determination in CryoSparc by Gold Standard Fourier Shell Correlation (GSFSC) is shown at the mid-bottom left. Corrected values give 3.30 Å overall resolution. Local resolution exceeds 2.5 Å, as shown on the bottom right. Particle orientational sampling is reasonably uniform as shown at the bottom left. Number of particles (images) holding a given azimuthal and elevation angle are indicated in the 2-dimensional histogram.
Extended Data Fig. 2 Tim domain construction and secondary structure assignments.
Domain construction and secondary structure assignments of Tim.
Extended Data Fig. 3 Cry domain construction and secondary structure assignments.
Domain construction and secondary structure assignments of Cry.
Extended Data Fig. 4 Key interactions at the Cry-Tim interface.
(a) Interactions between the bottom half of Tim ARM1-α1 with Cry. ARM1- α1 binds across Cry α14 and α17. The guanidinium of Cry R446 (on α17) forms π-cation interaction with Tim Y11. Tim Y11 participates in hydrophobic contacts with Cry P257, L443 and L447. On Tim α1, Tim P8 stacks against Cry α12 and Tim S15 hydrogen bonds to the backbone carbonyl of Cry A442. (b) In the β-hairpin connecting ARM1- α1 to ARM1- α2 E21 salt-bridges to R474 and C19 interacts with K445. (c) Interactions of the Cry protrusion motif. Tim R109, F113, and H110 interact with the protrusion motif of Cry. Tim ARM1-α7 H110 stacks with Cry H307. Four Met residues (M179, M181 M182, M185) in the ARM1-α9-α10 loop provide side-chain interactions with the tip of the Cry protrusion motif. The Cry α10 helix lengthens to residue A295 compared to the dark state and the following 300–306 residues interact directly with ARM1-α10. (d) Interactions of the Cry C-terminal lid and α20 helix. The restructured C-terminal lid interacts with residues on Tim ARM1- α2. For examples, I34 make a hydrophobic contact with I436 and E37 hydrogen bonds to the backbone of S434. At the end of ARM1- α2, Asn30 contacts the N-terminal end of Cry α20. A DSSO crosslink observed between Lys510 on Cry α20 and Lys41 on ARM1-α3 is fully consistent with the observed interface.
Extended Data Fig. 5 Changes in flavin binding pocket for Cry dark and bound states.
Comparison of the flavin pocket in Cry-Tim compared to in dark-state Cry (PDB 4GU5). (a) In Cry-Tim, Y250 and L251 interact with the FAD pocket and make space for the top of Tim ARM1-α3 to insert F58 behind the new conformation of the phosphate-binding loop. Q311 swivels away from Tim L4. (b) The FAD adenine ring of Cry-Tim shifts 1.5 Å relative to the unbound structure. Cry F318 shifts away from its original position and harbors helix α11 to compensate for the FAD adenine ring adjustment, following the protrusion motif. (c) Q. New diphosphate conformations couple to rearrangement of the Cry 262-to-265 loop.
Extended Data Fig. 6 Exit channel at the Cry-Tim interface and Cry secondary pocket.
(a) Putative exit channel for the additional 23 N-terminal residues of L-Tim. The first residue of Tim (M1) is colored as red. A solvent accessible channel at the interface between the two proteins is marked as the green arrow. (b) Electron density for the secondary binding pocket of CRY where antenna cofactors bind in photolyases and mCRY interact with other clock proteins (red box). Cry (yellow), Tim (periwinkle blue), Tim 1,209–1,219 (blue), Tim groove (navy blue). Although the pocket is well formed, there is no obvious density for additional moieties.
Extended Data Fig. 7 Tim peripheral helix and groove peptide fitting to the electron density map and associated Q-scores.
(a) Real-space electron density fitting metrics for the top scoring sequence assignments for the Tim peripheral helix. Real space density agreements were calculated in Q-scores and are shown above for sidechains and below for mainchains. One of the top scoring segments, residues 1209–1219 (top left) was also predicted by AlphaFold2.1 to interact with Cry at the position of the observed electron density, the model confidence pLDDT scores for this segment are given at the top right. (b) Real-space electron density fitting metrics for the top scoring sequence assignments for the Tim groove-binding polypeptide. Real space density agreements were calculated in Q-scores and are shown above for sidechains and below for mainchains. The Tim groove binding polypetide was tested in both directions.
Extended Data Fig. 8 Relative binding affinities between fragments of Tim and Cry H377L by the SWFTI assay.
(a) The Cry H377L variant acts as a constitutive light-activated state and enhances binding affinity for Tim by stabilizing the Tim-binding conformation of Cry16. H377L thereby increases Tim expression levels and facilitates detection of weaker binding variants. Mean ± standard error is shown for sample size = 3, from different biological replicates. One-way ANOVA with post-hoc Tukey HSD test was used to determine p values. (b) Pull down results of Cry-H377L with Tim-1133-1398. Cry-H377L interacts with the C-terminal regions of Tim. Gel lanes containing replicates or unrelated samples are not shown. (c) Quantification of the binding strength between Cry-H377L and Tim (1133–1398) compared to the negative control (Cry-H377L only with the same amount of resin). The binding strength is defined as the amount of Cry on the resin divided by the amount of Cry in the lysate sample. The negative control is normalized to 1. Mean ± standard error is shown for sample size = 3, from different biological replicates. Two-tailed unpaired t-test was used to determine p value. Binding affinities could not be derived from this experiment owing to the challenge of producing excessive Cry relative to Tim 1133–1398.
Extended Data Fig. 9 Comparisons of structural features in the Cry-Tim complex to those of CRY and TIM homologues.
(a) Structural comparison of Cry-Tim with mammalian CRY bound with mammalian PER. Tim 1209–1219 (blue) follows the backbone trace of mPER (CryΔ (yellow), Tim BD2 1209–1219 (blue)). 4CT0: Crystal Structure of Mouse Cryptochrome1 in Complex with Period2 (magenta). 4U8H: Crystal Structure of Mammalian Period-Cryptochrome Complex (green). 6OF7: Crystal structure of the CRY1-PER2 complex (orange red). (b) Structural comparison of Cry-Tim (Cry (yellow), Tim (periwinkle blue), Tim 1209–1219 (blue), Tim groove peptide (navy blue)) with the crystal structure of the N-terminal domain of human Timeless (PDB ID: 5MQI, salmon). Note that mammalian mTIM does not contain the N-terminal residues that bind into the Cry flavin pocket. (c) Structural superposition of Tim PAB (Parp-1 binding) domain and Parp-1 model with the structure of human TIM PAB bound to the PARP-1 catalytic domain (4XHU). Red box highlights the additional Tim α-helix (PAB-C-α4) that is incompatible with the interface formed by human PARP-1 and Tim PARP-1 binding. The Tim C region conserved across TIM proteins (residue 1051–1096) is also shown. Tim (this study, periwinkle blue), Drosophila Parp-1 (AlphaFold2.1 model: dark red) 4XHU (PAB:green, PARP-1:orange). (d) Superposition of Tim and Tof1 within the replisome.
Extended Data Fig. 10 Interactions between Tim and its homologs with extended polypeptides in their respective ARM grooves in relation to crosslinking map of Cry-Tim.
(a) Sequence alignment of top Tim structural homologs. Boxes outline the conserved R-XXX-N and RN residues within the ARM motifs that interact with the extended polypeptide chains of binding partners. Protein Data Bank (PDB) codes are shown on the left. (b) The extended peptide bound in the Tim groove resembles interactions found in other ARM-repeat proteins such as Importin-α1, β-catenin, and yeast TIM proteins. ARM-repeat proteins were structurally superimposed by their ARM-repeat cores to show the alignment of the extended peptides that bind in the crescent groove common to the family. For clarity only the groove peptides are displaced. Extended polypeptides found in yeast TOF1 (6SKL) and β-catenin (3IFQ) and Tim travel on the same lateral axis anti-parallel to the other importin- α, β-catenin, and Tim protein homologues. The PDB labels indicate the N terminus of the loop. 6SKL: Cryo-EM structure of the CMG Fork Protection Complex at a replication fork - Conformation 1 (lavender), 3IFQ: Interaction of plakoglobin and β-catenin with desmosomal cadherins (gold), 1JDH: Crystal structure of β-catenin and htcf-4 (purple), 3L3Q: Mouse importin α-pepTM NLS peptide complex (gray), 3KND: TPX2-importin-α complex (magenta), 1IQ1: Crystal structure of the importin-α(44–54)-importin-α(70–529) complex (salmon), 3UKX: Mouse importin-α:Bimax2 peptide complex (sky blue). (c) Map of DSSO lysine residue crosslinking defined by mass spectroscopy for the Cry-Tim complex. For the LysC+trypsin digested sample, although +2-charged peptides were excluded in the analysis, 59.79% sequence coverage for Cry and 49.81% for Tim were obtained. For the LysC+trypsin+GluC digested sample, 25.07% sequence coverage was obtained for Cry and 25.53% sequence coverage was obtained for Tim. Summary of the cross-links are given in the attached excel files and the raw data has been submitted to the PRIDE database.
Supplementary information
Supplementary Information
This file contains a methods overview schematic, DNA constructs, DNA sequences, protein sequences and Supplementary Table 1.
Supplementary Data
This file contains the raw data gel scans for Extended Fig. 8.
Supplementary Mass Spectrometry Data 1
Peptides from LysC–trypsin digestion of Cry–Tim complex.
Supplementary Mass Spectrometry Data 2
Peptides from LysC–trypsin–GluC digestion of Cry–Tim complex.
Supplementary Mass Spectrometry Data 3
Peptides from LysC–trypsin–GluC digestion of Cry–Tim complex, crosslinked peptides alone.
Supplementary Mass Spectrometry Data 4
Peptides from LysC–trypsin digestion of Cry–Tim complex, crosslinked peptides alone.
Supplementary Video 1
Overview of the Cry–Tim cryo-EM structure. The video depicts the cryo-EM density of the overall complex at different viewpoints and map contour levels with the superimposed model. Close-up views are provided for the FAD-binding pocket of Cry and the Tim N-terminal helix. A superposition with the dark-state structure of Cry is depicted to underscore the conformational changes Cry undergoes in the complex. The C-terminal Cry-binding domain of Tim and the Tim groove peptide are also highlighted.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, C., Feng, S., DeOliveira, C.C. et al. Cryptochrome–Timeless structure reveals circadian clock timing mechanisms. Nature 617, 194–199 (2023). https://doi.org/10.1038/s41586-023-06009-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06009-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.