Abstract
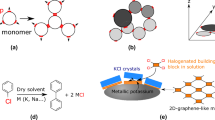
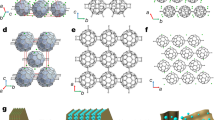
The two natural allotropes of carbon, diamond and graphite, are extended networks of sp3-hybridized and sp2-hybridized atoms, respectively1. By mixing different hybridizations and geometries of carbon, one could conceptually construct countless synthetic allotropes. Here we introduce graphullerene, a two-dimensional crystalline polymer of C60 that bridges the gulf between molecular and extended carbon materials. Its constituent fullerene subunits arrange hexagonally in a covalently interconnected molecular sheet. We report charge-neutral, purely carbon-based macroscopic crystals that are large enough to be mechanically exfoliated to produce molecularly thin flakes with clean interfaces—a critical requirement for the creation of heterostructures and optoelectronic devices2. The synthesis entails growing single crystals of layered polymeric (Mg4C60)∞ by chemical vapour transport and subsequently removing the magnesium with dilute acid. We explore the thermal conductivity of this material and find it to be much higher than that of molecular C60, which is a consequence of the in-plane covalent bonding. Furthermore, imaging few-layer graphullerene flakes using transmission electron microscopy and near-field nano-photoluminescence spectroscopy reveals the existence of moiré-like superlattices3. More broadly, the synthesis of extended carbon structures by polymerization of molecular precursors charts a clear path to the systematic design of materials for the construction of two-dimensional heterostructures with tunable optoelectronic properties.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are present in the paper and its Extended Data. The crystallographic data presented in this work are available through the Cambridge Crystallographic Data Centre (CCDC) referencing deposition no. 2151576. Further data are available from the corresponding authors upon reasonable request.
References
Hirsch, A. The era of carbon allotropes. Nat. Mater. 9, 868–871 (2010).
Liu, X. & Hersam, M. C. Interface characterization and control of 2D materials and heterostructures. Adv. Mater. 30, 1801586 (2018).
Andrei, E. Y. et al. The marvels of moiré materials. Nat. Rev. Mater. 6, 201–206 (2021).
Kroto, H. W., Heath, J. R., O’Brien, S. C., Curl, R. F. & Smalley, R. E. C60: buckminsterfullerene. Nature 318, 162–163 (1985).
David, W. I. F. et al. Crystal structure and bonding of ordered C60. Nature 353, 147–149 (1991).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Tanaka, M. & Yamanaka, S. Vapor-phase growth and structural characterization of single crystals of magnesium doped two-dimensional fullerene polymer Mg2C60. Cryst. Growth Des. 18, 3877–3882 (2018).
Hou, L. et al. Synthesis of a monolayer fullerene network. Nature 606, 507–510 (2022).
Dresselhaus, M. S., Dresselhaus, G. & Eklund, P. C. Science of Fullerenes And Carbon Nanotubes: Their Properties And Applications (Elsevier, 1996).
Park, S. et al. The phototransformation of C60 thin films on GaAs (100) studied by in situ Raman spectroscopy. J. Appl. Phys. 84, 1340–1345 (1998).
Burger, B., Winter, J. & Kuzmany, H. Dimer and cluster formation in C60 photoreaction. Z. Phys. B 101, 227–233 (1996).
Kuzmany, H., Matus, M., Burger, B. & Winter, J. Raman scattering in C60 fullerenes and fullerides. Adv. Mater. 6, 731–745 (1994).
Eda, G. et al. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 11, 5111–5116 (2011).
Wang, Y. et al. Optical absorption and photoluminescence in pristine and photopolymerized C60 solid films. Phys. Rev. B 51, 4547 (1995).
Kennes, D. M. et al. Moiré heterostructures as a condensed-matter quantum simulator. Nat. Phys. 17, 155–163 (2021).
Darlington, T. P. et al. Imaging strain-localized excitons in nanoscale bubbles of monolayer WSe2 at room temperature. Nat. Nanotechnol. 15, 854–860 (2020).
Hu, D. et al. Probing optical anisotropy of nanometer-thin van der Waals microcrystals by near-field imaging. Nat. Commun. 8, 1471 (2017).
Giri, A. & Hopkins, P. E. Spectral contributions to the thermal conductivity of C60 and the fullerene derivative PCBM. J. Phys. Chem. Lett. 8, 2153–2157 (2017).
Duda, J. C., Hopkins, P. E., Shen, Y. & Gupta, M. C. Exceptionally low thermal conductivities of films of the fullerene derivative PCBM. Phys. Rev. Lett. 110, 015902 (2013).
Giri, A. et al. Molecular tail chemistry controls thermal transport in fullerene films. Phys. Rev. Mater. 4, 065404 (2020).
Ong, W.-L. et al. Orientational order controls crystalline and amorphous thermal transport in superatomic crystals. Nat. Mater. 16, 83–88 (2017).
Wang, X., Liman, C. D., Treat, N. D., Chabinyc, M. L. & Cahill, D. G. Ultralow thermal conductivity of fullerene derivatives. Phys. Rev. B 88, 075310 (2013).
Braun, J. L., Olson, D. H., Gaskins, J. T. & Hopkins, P. E. A steady-state thermoreflectance method to measure thermal conductivity. Rev. Sci. Instrum. 90, 024905 (2019).
Rao, A. et al. Photoinduced polymerization of solid C60 films. Science 259, 955–957 (1993).
Lee, K. et al. Two‐dimensional fullerene assembly from an exfoliated van der Waals template. Angew. Chem. Int. Edn 130, 6233–6237 (2018).
Okada, S. et al. Direct microscopic analysis of individual C60 dimerization events: kinetics and mechanisms. J. Am. Chem. Soc. 139, 18281–18287 (2017).
Pekker, S. et al. Single-crystalline (KC60)n: a conducting linear alkali fulleride polymer. Science 265, 1077–1078 (1994).
Oszlányi, G., Baumgartner, G., Faigel, G. & Forró, L. Na4C60: an alkali intercalated two-dimensional polymer. Phys. Rev. Lett. 78, 4438 (1997).
Margadonna, S. et al. Li4C60: a polymeric fulleride with a two-dimensional architecture and mixed interfullerene bonding motifs. J. Am. Chem. Soc. 126, 15032–15033 (2004).
Riccò, M. et al. Unusual polymerization in the Li4C60 fulleride. Phys. Rev. B 72, 155437 (2005).
Heguri, S. & Kobayashi, M. The absence of a metallic phase in magnesium fullerides MgxC60 (1 < x ≤ 6). Solid State Commun. 150, 1489–1492 (2010).
Iwasa, Y. et al. New phases of C60 synthesized at high pressure. Science 264, 1570–1572 (1994).
Nunez-Regueiro, M., Marques, L., Hodeau, J.-L., Béthoux, O. & Perroux, M. Polymerized fullerite structures. Phys. Rev. Lett. 74, 278–281 (1995).
Sundqvist, B. Fullerenes under high pressures. Adv. Phys. 48, 1–134 (1999).
Moret, R., Launois, P., Persson, P.-A. & Sundqvist, B. First X-ray diffraction analysis of pressure polymerized C60 single crystals. Europhys. Lett. 40, 55 (1997).
Davydov, V. et al. Tetragonal polymerized phase of C60. Phys. Rev. B 58, 14786 (1998).
Chen, X. & Yamanaka, S. Single-crystal X-ray structural refinement of the ‘tetragonal’ C60 polymer. Chem. Phys. Lett. 360, 501–508 (2002).
Chen, X., Yamanaka, S., Sako, K., Inoue, Y. & Yasukawa, M. First single-crystal X-ray structural refinement of the rhombohedral C60 polymer. Chem. Phys. Lett. 356, 291–297 (2002).
Yamanaka, S. et al. Electron conductive three-dimensional polymer of cuboidal C60. Phys. Rev. Lett. 96, 076602 (2006).
Iwasa, Y. et al. Pressure-induced cross-linking of C60. Synth. Met. 70, 1407–1408 (1995).
CrysAlis PRO (Agilent Technologies Ltd, Yarnton, Oxfordshire, UK, 2014).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Sheldrick, G. M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015).
Holder, C. F. & Schaak, R. E. Tutorial on powder X-ray diffraction for characterizing nanoscale materials. ACS Nano 13, 7359–7365 (2019).
Ho, P. K., Kim, J.-S., Tessler, N. & Friend, R. H. Photoluminescence of poly(p-phenylenevinylene)–silica nanocomposites: evidence for dual emission by Franck–Condon analysis. Chem. Phys. 115, 2709–2720 (2001).
Kang, K., Koh, Y. K., Chiritescu, C., Zheng, X. & Cahill, D. G. Two-tint pump–probe measurements using a femtosecond laser oscillator and sharp-edged optical filters. Rev. Sci. Instrum. 79, 114901 (2008).
Cahill, D. G. Analysis of heat flow in layered structures for time-domain thermoreflectance. Rev. Sci. Instrum. 75, 5119–5122 (2004).
Braun, J. L. & Hopkins, P. E. Upper limit to the thermal penetration depth during modulated heating of multilayer thin films with pulsed and continuous wave lasers: a numerical study. J. Appl. Phys. 121, 175107 (2017).
Wang, X., Ho, V., Segalman, R. A. & Cahill, D. G. Thermal conductivity of high-modulus polymer fibers. Macromolecules 46, 4937–4943 (2013).
Braun, J. L., Szwejkowski, C. J., Giri, A. & Hopkins, P. E. On the steady-state temperature rise during laser heating of multilayer thin films in optical pump–probe techniques. J. Heat Transfer 140, 052801 (2018).
Sun, H. COMPASS: an ab initio force-field optimized for condensed-phase applications—overview with details on alkane and benzene compounds. J. Phys. Chem. B 102, 7338–7364 (1998).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Schelling, P. K., Phillpot, S. R. & Keblinski, P. Comparison of atomic-level simulation methods for computing thermal conductivity. Phys. Rev. B 65, 144306 (2002).
Acknowledgements
This work was primarily funded by the Columbia MRSEC on Precision-Assembled Quantum Materials (PAQM) under award number DMR-2011738, and the Air Force Office of Scientific Research under grant FA9550-22-1-0389. Surface characterization of graphullerene was supported by the NSF CAREER award DMR-1751949 (X.R.). Calorimetry measurements were supported by the NSF award CBET-2017198 (X.R.). C.N. acknowledges support from the Office of Naval Research Award N00014-20-1-2477, and thanks S. Buckler and D. Buckler for their generous support. M.M., C.J.D., A.G. and P.E.H. acknowledge support from the Office of Naval Research, grant numbers N00014-20-1-2686 and N00014-21-1-2622. Infrared nano-imaging experiments are supported under Energy Frontier Research Center on Programmable Quantum Materials funded by the US Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES), under award DE-SC0019443. E.M. was supported in part by the Yad Hanadiv and Weizmann Women in Science Award for postdoctoral fellowship support. A.M.E. was supported by the Schmidt Science Fellows, in partnership with the Rhodes Trust. M.R and P.K. were supported by Army Research Office under Award W911NF-18-1-0366. R.A.W. and A.K.B. were supported by Arnold O. Beckman Fellowships in Chemical Sciences. T.H. acknowledges the postdoctoral fellowship support of JSPS Overseas Research Fellowships. X-ray diffraction, electron microscopy, AFM, zeta-potential and TGA measurements were performed in the Shared Materials Characterization Laboratory at Columbia University. We thank L. M. Campos and E. M. Churchill for their assistance with the DSC measurements. Raman spectroscopy measurements were supported by the Barnard College Department of Chemistry and Office of the Provost. We thank N. Cislo for assistance with early Raman spectroscopy measurements.
Author information
Authors and Affiliations
Contributions
Conceptualization: E.M., J.Y., M.L.S., C.N., X.R. Methodology and investigation: E.M., A.M.E., S.T.B., A.K.B., R.A.W. Electron microscopy: A.M.E., A.Z. Device fabrication and electrical transport: M.Rezaee., P.K. Photoluminescence: T.H., X.Z., T.P.D, N.F.-M., P.J.S. s-SNOM: D.J.R., D.N.B. Raman spectroscopy: M.Reza., A.C.C. Thermal properties: M.M., C.J.D., A.G., P.E.H. Writing: E.M., A.M.E., M.L.S., C.N., X.R. Supervision: M.L.S., C.N., X.R.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Andreas Hirsch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Electrical transport properties of (Mg4C60)∞.
Electrical conductivity (σ) of (Mg4C60)∞ versus temperature.
Extended Data Fig. 2 Powder X-ray diffraction of C60 polymers.
PXRD pattern of graphullerite (C60)∞, (Mg4C60)∞, and molecular C60. The red vertical lines represent the calculated peak positions for (Mg4C60)∞, assuming a preferred orientation and 1.8 Å expansion along the a axis, the stacking direction.
Extended Data Fig. 3 Raman spectra and thermal stability of C60 polymers.
a, Raman spectra of (Mg4C60)∞, graphullerite (C60)∞, and mechanically exfoliated bilayer graphullerene (2L). The purple vertical line denotes the pentagonal pinch mode for molecular C60 (1,469 cm–1). The Ag(2) mode of graphullerite and graphullerene may overlap with the broad Hg(7) mode at 1,420 cm–1. b, Raman spectra of graphullerite after annealing at 400 and 600 °C for 1 h. The Ag(2) mode characteristic of molecular C60 appears at 1,469 cm–1 after annealing at 600 °C.
Extended Data Fig. 4 Calorimetry and thermogravimetric analysis of C60 polymers.
a, DSC traces of (Mg4C60)∞ and graphullerite crystals. The heat flow was measured as a function of temperature at a constant temperature ramp of 10 °C min–1 during heating and 5 °C min–1 during cooling with a 50 ml /min−1 nitrogen cell purge flow. b, Weight loss as a function of temperature for molecular C60 and (Mg4C60)∞ under N2 at a constant temperature ramp of 10 °C min–1, measured by TGA. The 6% increase in the (Mg4C60)∞ data is presumably due to the formation of MgO.
Extended Data Fig. 5 Powder X-ray diffraction of graphullerite after thermal treatment.
The PXRD patterns of graphullerite after annealing in vacuo for 1 h at different temperatures, along with the patterns of (Mg4C60)∞ and molecular C60. Above 500 °C the covalently bonded sheets depolymerize, and molecular C60 peaks emerge and become more intense above 800 °C.
Extended Data Fig. 6 Zeta potential of graphullerite suspended in isopropanol.
Inset: image of the suspension used for this measurement.
Extended Data Fig. 7 Transmission electron microscopy image of graphullerene.
The layered structure of graphullerene is apparent in this low-magnification image. Our best estimate for the number of layers within the selected area (black box) is 2–4 based on how the contrast increases stepwise from the edge of the flake towards the selected area.
Extended Data Fig. 8 Photoluminescence of (Mg4C60)∞.
a, The open circles represent the experimental data and the solid line is the fitting result based on the Franck–Condon model. b, PL intensity as a function of excitation laser power.
Extended Data Fig. 9 Thermal property characterization.
a, Thermal conductivity measurements on graphullerite were conducted using a fibre-based SSTR set-up similar to the schematic shown. b, The inverse of thermal conductivity versus the inverse of the simulation domain length.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meirzadeh, E., Evans, A.M., Rezaee, M. et al. A few-layer covalent network of fullerenes. Nature 613, 71–76 (2023). https://doi.org/10.1038/s41586-022-05401-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05401-w
This article is cited by
-
Structure and properties of graphullerene: a semiconducting two-dimensional C60 crystal
npj Computational Materials (2023)
-
On-surface synthesis of a doubly anti-aromatic carbon allotrope
Nature (2023)
-
Current Understanding on the Unique Relaxation Dynamics of Sub-nanometer Materials and Their Structure-Property Relationships
Chemical Research in Chinese Universities (2023)
-
Highly in-plane anisotropic optical properties of fullerene monolayers
Science China Physics, Mechanics & Astronomy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.