Abstract
Most quality control pathways target misfolded proteins to prevent toxic aggregation and neurodegeneration1. Dimerization quality control further improves proteostasis by eliminating complexes of aberrant composition2, but how it detects incorrect subunits remains unknown. Here we provide structural insight into target selection by SCF–FBXL17, a dimerization-quality-control E3 ligase that ubiquitylates and helps to degrade inactive heterodimers of BTB proteins while sparing functional homodimers. We find that SCF–FBXL17 disrupts aberrant BTB dimers that fail to stabilize an intermolecular β-sheet around a highly divergent β-strand of the BTB domain. Complex dissociation allows SCF–FBXL17 to wrap around a single BTB domain, resulting in robust ubiquitylation. SCF–FBXL17 therefore probes both shape and complementarity of BTB domains, a mechanism that is well suited to establish quality control of complex composition for recurrent interaction modules.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The atomic coordinates of the CUL1–SKP1–FBXL17–KEAP1(V99A) complex has been deposited to PDB with accession number 6WCQ. The respective cryo-EM map has been deposited to the Electron Microscopy Data Bank with accession number EMD-21617. The atomic coordinates of the X-ray crystal structures have been deposited to the PDB with the following accession numbers: 6W66 (KEAP1(S172A/F64A)–FBXL17–SKP1 complex), 6W67 (KEAP1(S172A)), 6W68 (KEAP1(S172A/V98A)) and 6W69 (KEAP1(S172A/F64A)).
References
Balchin, D., Hayer-Hartl, M. & Hartl, F. U. In vivo aspects of protein folding and quality control. Science 353, aac4354 (2016).
Mena, E. L. et al. Dimerization quality control ensures neuronal development and survival. Science 362, eaap8236 (2018).
Gordley, R. M., Bugaj, L. J. & Lim, W. A. Modular engineering of cellular signaling proteins and networks. Curr. Opin. Struct. Biol. 39, 106–114 (2016).
Ji, A. X. & Privé, G. G. Crystal structure of KLHL3 in complex with Cullin3. PLoS ONE 8, e60445 (2013).
Zhuang, M. et al. Structures of SPOP–substrate complexes: insights into molecular architectures of BTB–Cul3 ubiquitin ligases. Mol. Cell 36, 39–50 (2009).
Cleasby, A. et al. Structure of the BTB domain of Keap1 and its interaction with the triterpenoid antagonist CDDO. PLoS ONE 9, e98896 (2014).
Ghetu, A. F. et al. Structure of a BCOR corepressor peptide in complex with the BCL6 BTB domain dimer. Mol. Cell 29, 384–391 (2008).
McGourty, C. A. et al. Regulation of the CUL3 ubiquitin ligase by a calcium-dependent co-adaptor. Cell 167, 525–538 (2016).
Werner, A. et al. Cell-fate determination by ubiquitin-dependent regulation of translation. Nature 525, 523–527 (2015).
Jin, L. et al. Ubiquitin-dependent regulation of COPII coat size and function. Nature 482, 495–500 (2012).
Furukawa, M. & Xiong, Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3–Roc1 ligase. Mol. Cell. Biol. 25, 162–171 (2005).
Wakabayashi, N. et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 35, 238–245 (2003).
Louis-Dit-Picard, H. et al. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat. Genet. 44, 456–460 (2012).
Maerki, S. et al. The Cul3–KLHL21 E3 ubiquitin ligase targets aurora B to midzone microtubules in anaphase and is required for cytokinesis. J. Cell Biol. 187, 791–800 (2009).
Sumara, I. et al. A Cul3-based E3 ligase removes Aurora B from mitotic chromosomes, regulating mitotic progression and completion of cytokinesis in human cells. Dev. Cell 12, 887–900 (2007).
Duan, S. et al. FBXO11 targets BCL6 for degradation and is inactivated in diffuse large B-cell lymphomas. Nature 481, 90–93 (2012).
Tan, M. K., Lim, H. J., Bennett, E. J., Shi, Y. & Harper, J. W. Parallel SCF adaptor capture proteomics reveals a role for SCFFBXL17 in NRF2 activation via BACH1 repressor turnover. Mol. Cell 52, 9–24 (2013).
Yamamoto, M., Kensler, T. W. & Motohashi, H. The KEAP1–NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 98, 1169–1203 (2018).
Zheng, N. et al. Structure of the Cul1–Rbx1–Skp1–F boxSkp2 SCF ubiquitin ligase complex. Nature 416, 703–709 (2002).
Xing, W. et al. SCFFBXL3 ubiquitin ligase targets cryptochromes at their cofactor pocket. Nature 496, 64–68 (2013).
Schulman, B. A. et al. Insights into SCF ubiquitin ligases from the structure of the Skp1–Skp2 complex. Nature 408, 381–386 (2000).
Bhattacharyya, M. et al. Molecular mechanism of activation-triggered subunit exchange in Ca2+/calmodulin-dependent protein kinase II. eLife 5, e13405 (2016).
Pierce, N. W. et al. Cand1 promotes assembly of new SCF complexes through dynamic exchange of F box proteins. Cell 153, 206–215 (2013).
Reitsma, J. M. et al. Composition and regulation of the cellular repertoire of SCF ubiquitin ligases. Cell 171, 1326–1339 (2017).
Liu, X. et al. Cand1-mediated adaptive exchange mechanism enables variation in F-box protein expression. Mol. Cell 69, 773–786 (2018).
Wu, S. et al. CAND1 controls in vivo dynamics of the cullin 1–RING ubiquitin ligase repertoire. Nat. Commun. 4, 1642 (2013).
Zemla, A. et al. CSN- and CAND1-dependent remodelling of the budding yeast SCF complex. Nat. Commun. 4, 1641 (2013).
Duda, D. M. et al. Structural insights into NEDD8 activation of cullin–RING ligases: conformational control of conjugation. Cell 134, 995–1006 (2008).
Kabsch, W. Xds. Acta Crystallogr. D 66, 125–132 (2010).
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr. D 69, 1204–1214 (2013).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 67, 235–242 (2011).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
DiMaio, F. et al. Improved low-resolution crystallographic refinement with Phenix and Rosetta. Nat. Methods 10, 1102–1104 (2013).
Morin, A. et al. Collaboration gets the most out of software. eLife 2, e01456 (2013).
Grimsley, G. R., Huyghues-Despointes, B. M., Pace, C. N. & Scholtz, J. M. Preparation of urea and guanidinium chloride stock solutions for measuring denaturant-induced unfolding curves. CSH Protoc. 2006, pdb.prot4241 (2006).
McDonald, S. K. & Fleming, K. G. Aromatic side chain water-to-lipid transfer free energies show a depth dependence across the membrane normal. J. Am. Chem. Soc. 138, 7946–7950 (2016).
Tivol, W. F., Briegel, A. & Jensen, G. J. An improved cryogen for plunge freezing. Microsc. Microanal. 14, 375–379 (2008).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Schorb, M., Haberbosch, I., Hagen, W. J. H., Schwab, Y. & Mastronarde, D. N. Software tools for automated transmission electron microscopy. Nat. Methods 16, 471–477 (2019).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Biyani, N. et al. Focus: The interface between data collection and data processing in cryo-EM. J. Struct. Biol. 198, 124–133 (2017).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Scheres, S. H. W. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods 9, 853–854 (2012).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Acknowledgements
We thank C. Nixon, S. Costello and S. Marqusee for their generous help with circular dichroism; members of the A. Martin laboratory for their help with FRET; L. Nocka for help with SEC–MALS; F. Rodriguez Perez for help with R; A. Patel and D. Toso for help with graphene oxide grid preparation and cryo-EM data collection, respectively; J. Holton and G. Meigs at the Advanced Light Source Beamline 8.3.1 for assistance with data collection; J. Schaletzky for her comments on the manuscript; and members of the Rape, Nogales and Kuriyan laboratories for discussion and suggestions. Beamline 8.3.1 at the Advanced Light Source is operated by the University of California Office of the President, Multicampus Research Programs and Initiatives grant MR-15-328599, the National Institutes of Health (R01 GM124149 and P30 GM124169), Plexxikon and the Integrated Diffraction Analysis Technologies program of the US Department of Energy Office of Biological and Environmental Research. The Advanced Light Source (Berkeley) is a national user facility operated by Lawrence Berkeley National Laboratory on behalf of the US Department of Energy under contract number DE-AC02-05CH11231, Office of Basic Energy Sciences. E.L.M. was funded by an NSF predoctoral scholarship (ID 2013157149). B.J.G. was supported by fellowships from the Swiss National Science Foundation (projects P300PA_160983, P300PA_174355). P.J. was funded by a Siebel Institute postdoctoral fellowship. D.A. was funded by an NIH F32 postdoctoral fellowship. E.N., J.K. and M.R. are investigators with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
E.L.M., B.G.L. and D.A. purified recombinant proteins and performed SEC, SEC–MALS, CD and FRET experiments. E.L.M., B.G.L. and C.L.G. performed crystallization experiments. B.J.G. and E.L.M. performed cryo-EM experiments. P.J. performed immunoprecipitation, degradation and mass spectrometry analyses. E.L.M. and P.J. performed in vitro binding assays. P.J. and E.L.M. performed in vitro ubiquitylation assays. P.J. and D.A. performed in vitro titration assays. All authors interpreted the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.R. and J.K. are founders and consultants of Nurix, a biotechnology company working in the ubiquitin field.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Variant BTB domains of KEAP1 are recognized by SCFFBXL17.
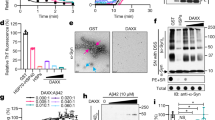
a, Mutations in KEAP1’s BTB domain result in efficient recognition by SCFFBXL17. The same mutations as in Fig. 1a were introduced into the KEAP1S172A variant that had previously been used for crystallization. 35S-labelled double mutants, but not the KEAP1S172A single mutant, were retained by immobilized MBPFBXL17, as detected by gel electrophoresis and autoradiography. This experiment was performed once. b, SCFFBXL17 strongly prefers mutant over wild-type BTB domains. Increasing concentrations of MBPFBXL17 were immobilized on amylose beads and incubated with 100 nM wild-type or mutant KEAP1. Depletion of KEAP1 from the supernatant was measured by quantitative LiCor imaging of Coomassie-stained SDS–PAGE gels. The affinity of FBXL17 to wild-type KEAP1 was too low to be determined reliable by this method. Three independent experiments were performed with similar results. c, Mutant BTB domains form homodimers in vitro. Recombinant BTB domains of KEAP1, KEAP1F64A, and KEAPV98A (MW about 15 kDa) were analysed by size exclusion chromatography detecting A280. Expected position of BTB dimer versus monomer, as well as of control proteins with known MW are shown on top. Three independent experiments were performed with similar results. d, BTB domains of wild-type KEAP1 and mutant KEAP1F64A form homodimers in solution, as determined by SEC-MALS. This experiment was performed twice. e, Mutant BTB domains unfold via an intermediate species. Wild-type or mutant BTB domains of KEAP1 (0.04 mg/ml) were incubated with various concentrations of urea, equilibrated overnight, and their resulting secondary structure was monitored by ellipticity at 222 nm using circular dichroism. The experiment was performed once for the mutants and twice for the wild-type KEAP1. f, The intermediate seen in the unfolding of KEAP1V98A probably reflects local conformational changes, rather than monomerization. Urea-dependent unfolding curves for the BTB domain of KEAP1V98A were repeated at tenfold higher BTB concentration (low: 0.04 mg/ml; high: 0.4 mg/ml); only the second transition shifted to higher urea concentrations, identifying it as a dimer-unfolded transition. This experiment was performed once. g. Mutation of Phe64 or Val98 to Ala in the BTB domain of KEAP1 reduces contacts between helices of two interacting subunits of the KEAP1 dimer.
Extended Data Fig. 2 Cryo-EM data collection and processing.
a, Representative micrograph (graphene oxide coated grid, imaged using a Talos Arctica and a Gatan K3 camera) showing CUL1-SKP1-FBXL17-KEAP1V98A particles. b, Resolution estimation using the FSC = 0.143 criterion50 indicates an overall resolution of 8.5 Å for the cryo-EM reconstruction. c. Data processing scheme. Datasets were initially processed independently and then combined for the final refinement. EM volumes are shown in grey and orientation distributions are given for intermediate refinement steps. The final reconstruction is shown with and without sharpening applied, and additionally colored by local resolution (determined using RELION3). d, e, Initial model generation. We originally obtained an initial reference by generating a low-resolution volume with the overall shape of the complex observed in 2D class averages (d) and later also verified this solution using CRYOSPARC2 ab initio model generation (e; see Methods for details).
Extended Data Fig. 3 SCFFBXL17 binds the BTB domain, but its active site is next to the Kelch repeats of KEAP1.
a, Elution profile of the SCFFBXL17-KEAP1V99A complex by size exclusion chromatography detecting A280. Control proteins with known MW are shown on top. This experiment was performed three times. b, Cryo-EM density map of the SCFFBXL17-KEAP1V98A complex. Dark grey: CUL11–450; light grey: SKP1; orange: FBXL17; blue: KEAP1. c, Despite an overlap in binding sites, CUL3 does not compete with SCFFBXL17 for substrate ubiquitylation. 35S-labelled wild-type or mutant KEAP1 were ubiquitylated by SCFFBXL17 in reticulocyte lysate either in the presence or absence of a CUL3 variant shown to bind BTB proteins. Ubiquitylated KEAP1 was detected by gel electrophoresis and autoradiography. Three independent experiments were performed with similar results. d, SCFFBXL17 ubiquitylates full-length BTB proteins with Kelch repeats better than isolated BTB domains. 35S-labelled full-length KEAP1F64A, the BTB domain of KEAP1F64A, full-length KLHL12V50A, or the BTB domain of KLHL12V50A were incubated in reticulocyte lysate with recombinant FBXL17, and ubiquitylation was detected by gel electrophoresis and autoradiography. Two independent experiments were performed with similar results. e, Full-length BTB proteins or isolated BTB domains bind similarly well to FBXL17. 35S-labelled full-length BTB proteins or isolated BTB domains, as indicated on the right, were incubated with immobilized MBPFBXL17 and bound proteins were detected by gel electrophoresis and autoradiography. Two independent experiments were performed with similar results.
Extended Data Fig. 4 Structural features of the SKP1/FBXL17-BTB complex.
a, Elution profile of the SKP1/FBXL17-BTB(KEAP1F64A) complex by size exclusion chromatography detecting A280. Control proteins with known MW are shown on top. This experiment was performed three times. b, FBXL17 binds to SKP1 via its F-box domain, in a manner highly similar to the LRR-domain containing F-box proteins SKP2 and FBXL320,21. The structures of SKP1-FBXL17, SKP1-SKP2, and SKP1-FBXL3 were aligned via SKP1. FBXL17 is shown in orange, SKP2 in magenta, and FBXL3 in yellow. c, FBXL17 uses conserved residues in its F-box to bind SKP1. The highlighted residues in FBXL17 (orange) that bind SKP1 (grey) were adopted from ref. 21. d, The substrate binding LRR domain of FBXL17 is longer and more curved than the LRR domains of SKP2 or FBXL3. Complexes were aligned via SKP1 (FBXL17, orange; SKP2, magenta; FBXL3, yellow). e, Structural models of BTB-FBXL17 complexes, using BTB domains that are similar in shape, but distinct in sequence. All complexes between FBXL17 and these confirmed substrates2 can be formed without steric clashes. f, SKP1 and Elongin C, which also adopt BTB folds, cannot be bound to FBXL17, due to steric clashes shown in the insets.
Extended Data Fig. 5 Validation of SKP1/FBXL17-BTB structure through FBXL17 mutations.
a, Single mutations of FBXL17 rarely affect co-translational recognition of KEAP1 in cells. FBXL17FLAG mutants were affinity-purified from 293T cells that also expressed MYCSKP1, HAKEAP1, and dominant negative CUL1 to prevent degradation of the BTB protein. Bound proteins were detected by gel electrophoresis and western blotting. Red, mutations that abolish binding to FBXL17; orange, mutations that weaken binding to FBXL17; green, wild-type FBXL17; black: mutations that had no effect on KEAP1 binding. This experiment was performed once. b, Single mutations of FBXL17 rarely interfere with the proteasomal degradation of KEAP1. 293T cells were transfected with HAKEAP1 and either wild-type or mutant FBXL17FLAG, as denoted on the right, MYCSKP1, and dominant-negative CUL1 (dnCUL1), as indicated. The abundance of KEAP1 was monitored by gel electrophoresis and αHA-Western blotting. This experiment was performed once. c, The CTH is required, but not sufficient, for BTB recognition by SCFFBXL17. Immobilized recombinant MBP, MBPFBXL17, MBPFBXL17ΔCTH, or CTHMBP were incubated with 35S-labelled fused dimers of the BTB domains of KLHL12 (green) and KEAP1 (orange). Bound proteins were detected by gel electrophoresis and autoradiography. This experiment was performed once. d, The CTH is required for in vitro ubiquitylation of mutant KEAP1. Recombinant FBXL17-SKP1 or FBXL17ΔCTH-SKP1 were added to reticulocyte lysate after the synthesis of either wild-type or mutant KEAP1. Reticulocyte lysate contains all other components required for in vitro ubiquitylation through SCFFBXL17. Unmodified and ubiquitylated KEAP1 were detected by gel electrophoresis and autoradiography. This experiment was performed once.
Extended Data Fig. 6 Validation of SKP1/FBXL17-BTB structure through KEAP1 mutations.
a, Single mutations in KEAP1 do not inhibit the co-translational SCFFBXL17-dependent degradation of the BTB protein. 293T cells were transfected with either wild-type (left three lanes) or mutant HAKEAP1 (right two lanes; mutations denoted on the right), as well as MYCSKP1, FBXL17FLAG and dominant negative CUL1 (dnCUL1), as indicated on top. KEAP1 levels were monitored by gel electrophoresis and αHA western blotting. This experiment was performed once. b, Single mutation of residues in KEAP1 at the interface with FBXL17 do not inhibit co-translational binding of the BTB protein to SCFFBXL17. 293T cells were transfected with wild-type or mutant HAKEAP1, MYCSKP1, FBXL17FLAG, and dominant-negative CUL1 (dnCUL1). FBXL17FLAG was affinity-purified and bound proteins were detected by gel electrophoresis and western blotting. This experiment was performed once. c, Ala109 (red stick) in KEAP1 (blue) is positioned further from FBXL17 compared to the corresponding A60 residue in KLHL12 (green). KEAP1 and KLHL12 BTB domains were overlain bound to FBXL17 (KEAP1, actual structure; KLHL12, model).
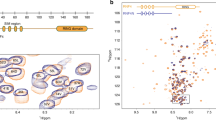
Extended Data Fig. 7 Sequence alignment of BTB domains.
Residues involved in BTB dimerization are marked by a blue dot; residues at the interface between the BTB domain and FBXL17 are marked by an orange dot; residues at the interface between the BTB domain and CUL3 are marked by a magenta dot. Sites of mutations used for X-ray crystallography or electron microscopy are marked by a red star.
Extended Data Fig. 8 Binding and destabilization of BTB dimers by SCFFBXL17.
a, A FRET-based assay to monitor BTB dimer formation. Blue curve: The BTB domain of KEAP1F64A was labelled with Alexa 555, then denatured and refolded. Red curve: The BTB domain of KEAP1F64A was labelled with Alexa 647, then denatured and refolded. Green curve: Two separate BTB domain pools of KEAP1F64A were labelled with either Alexa 555 or Alexa 647, mixed in equimolar concentrations, denatured, and then refolded. About 50% of dimers are labelled with distinct fluorophores in each BTB subunit, giving rise to donor fluorescence quenching and acceptor emission as indication of FRET. Three independent experiments were performed with similar results. b, KEAP1F64A dimers dissociate very slowly and inefficiently. BTB domains of KEAP1F64A were labelled with either Alexa 555 or Alexa 647, respectively. The labelled BTB domains were then mixed, incubated overnight, and analysed for FRET that results from stochastic rebinding of BTB monomers, leading to formation of BTB dimers containing one subunit labelled with Alexa 555 and the other subunit labelled with Alexa 647. However, in comparison to complex reformation by refolding (see above), little FRET was detected. This experiment was performed twice. c, FBXL17 can modulate BTB complex composition in vitro. The KLHL12 locus was tagged with a 3xFLAG epitope by CRISPR/Cas9-mediated genome editing. Endogenous KLHL123xFLAG complexes were affinity-purified from 293T cells and incubated with recombinant MBP (control), FBXL17, or inactive FBXL17ΔCTH. Proteins that remained bound to KLHL123xFLAG were determined by mass spectrometry. d, Overexpression of FBXL17ΔFBOX, which can bind but not ubiquitylate BTB proteins, prevents BTB heterodimerization. The endogenous KLHL123xFLAG was affinity-purified either in the presence or absence of FBXL17ΔFbox, and bound proteins were determined by mass spectrometry. e, FBXL17 can bind BTB dimers. FLAGKLHL12 was affinity-purified from 293T cells also expressing MYCKLHL12 and FBXL17HA. FLAGKLHL12 complexes were eluted with FLAG-peptide and FBXL17HA-containing complexes were then purified over αHA-agarose. Bound MYCKLHL12, indicative of FBXL17 associating with KLHL12 dimers, was then detected by western blotting. This experiment was performed once. f, Binding of FBXL17 to BTB dimers requires its CTH to be disengaged from its binding site at the BTB dimer interface. A structural model of a KEAP1 BTB dimer bound to FBXL17 was generated using the KEAP1F64A dimer and the FBXL17-KEAP1F64A complex structures. Clashes are predominantly at the CTH of FBXL17. g, Residues of FBXL17, which in the structural model of a FBXL17-BTB dimer complex are in proximity to the leaving BTB subunit, contribute to stable substrate recognition. Indicated FBXL17 residues at the interface with the leaving BTB subunit (above) were mutated in the sensitized background of the FBXL17C680D variant and analysed for binding to endogenous BTB proteins by affinity purification and western blotting. This experiment was performed once.
Extended Data Fig. 9 The N-terminal β-strand is important for BTB complex formation and recognition.
a, Chimeric KLHL12 with β-strand and adjacent dimer interface residues of KEAP1, but not wild-type KLHL12, forms heterodimers with KEAP1 in vivo. 293T cells were transfected with KLHL12FLAG (wild-type or chimaera) and KLHL12HA or HAKEAP1, as indicated. KLHL12FLAG variants were immunoprecipitated and bound proteins detected by gel electrophoresis and western blotting. This experiment was performed once. b, BTB heterodimers are inactive in signalling. 293T cells were transfected with FLAG-tagged wild-type KLHL12, chimeric KLHL12, or wild-type KEAP1. The FLAG-tagged BTB proteins were affinity-purified and bound endogenous targets of KLHL12 (SEC31, PEF1, ALG2) or KEAP1 (NRF2) were detected by western blotting. This experiment was performed once. c, A chimeric KLHL12 that contains helix and β-strand residues of KEAP1 efficiently heterodimerizes with KEAP1, yet fails to bind substrates of either KLHL12 or KEAP1. KLHL12FLAG, chimeric KLHL12FLAG or KEAP1FLAG were affinity-purified from 293T cells and bound proteins were determined by CompPASS mass spectrometry. This experiment was performed in two technical replicates with similar results.
Extended Data Fig. 10 The amino-terminal β-strand of BTB domains serves as a molecular barcode for functional dimerization.
Sequence alignment of amino-terminal β-strands across human BTB domains shows divergence, indicative of rapid evolution of this protein sequence.
Supplementary information
Supplementary Information
Original Data 1: Contains original uncropped Western blots, part 1.
Supplementary Information
Original Data 2: Contains original uncropped Western blots, part 2.
Rights and permissions
About this article
Cite this article
Mena, E.L., Jevtić, P., Greber, B.J. et al. Structural basis for dimerization quality control. Nature 586, 452–456 (2020). https://doi.org/10.1038/s41586-020-2636-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2636-7
This article is cited by
-
Structural insights into the ubiquitylation strategy of the oligomeric CRL2FEM1B E3 ubiquitin ligase
The EMBO Journal (2024)
-
FBXL17/spastin axis as a novel therapeutic target of hereditary spastic paraplegia
Cell & Bioscience (2022)
-
Transcriptome, metabolome and suppressor analysis reveal an essential role for the ubiquitin-proteasome system in seedling chloroplast development
BMC Plant Biology (2022)
-
Co-translational assembly orchestrates competing biogenesis pathways
Nature Communications (2022)
-
Understanding the Xylooligosaccharides Utilization Mechanism of Lactobacillus brevis and Bifidobacterium adolescentis: Proteins Involved and Their Conformational Stabilities for Effectual Binding
Molecular Biotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.