Abstract
Systemic lupus erythematosus (SLE) is an incurable autoimmune disease characterized by autoantibody deposition in tissues such as kidney, skin and lungs. Notably, up to 75% of patients with SLE experience neuropsychiatric symptoms that range from anxiety, depression and cognitive impairment to seizures and, in rare cases, psychosis—collectively this is referred to as central nervous system (CNS) lupus1,2,3,4. In some cases, certain autoantibodies, such as anti-NMDAR or anti-phospholipid antibodies5,6, promote CNS lupus. However, in most patients, the mechanisms that underlie these symptoms are unknown. CNS lupus typically presents at lupus diagnosis or within the first year, suggesting that early factors contributing to peripheral autoimmunity may promote CNS lupus symptoms. Here we report behavioural phenotypes and synapse loss in lupus-prone mice that are prevented by blocking type I interferon (IFN) signalling. Furthermore, we show that type I IFN stimulates microglia to become reactive and engulf neuronal and synaptic material in lupus-prone mice. These findings and our observation of increased type I IFN signalling in post-mortem hippocampal brain sections from patients with SLE may instruct the evaluation of ongoing clinical trials of anifrolumab7, a type I IFN-receptor antagonist. Moreover, identification of IFN-driven microglia-dependent synapse loss, along with microglia transcriptome data, connects CNS lupus with other CNS diseases and provides an explanation for the neurological symptoms observed in some patients with SLE.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Popescu, A. & Kao, A. H. Neuropsychiatric systemic lupus erythematosus. Curr. Neuropharmacol. 9, 449–457 (2011)
Feinglass, E. J., Arnett, F. C., Dorsch, C. A., Zizic, T. M. & Stevens, M. B. Neuropsychiatric manifestations of systemic lupus erythematosus: diagnosis, clinical spectrum, and relationship to other features of the disease. Medicine 55, 323–339 (1976)
van Dam, A. P. Diagnosis and pathogenesis of CNS lupus. Rheumatol. Int. 11, 1–11 (1991)
Hanly, J. G. et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 56, 265–273 (2007)
DeGiorgio, L. A. et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 7, 1189–1193 (2001)
Sanna, G. et al. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J. Rheumatol. 30, 985–992 (2003)
Furie, R. et al. Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 69, 376–386 (2017)
Berland, R. et al. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity 25, 429–440 (2006)
Das, A. et al. Follicular dendritic cell activation by TLR ligands promotes autoreactive B cell responses. Immunity 46, 106–119 (2017)
Lynch, M. A. The multifaceted profile of activated microglia. Mol. Neurobiol. 40, 139–156 (2009)
Crupi, R. et al. Reduced adult neurogenesis and altered emotional behaviors in autoimmune-prone B-cell activating factor transgenic mice. Biol. Psychiatry 67, 558–566 (2010)
Mondal, T. K., Saha, S. K., Miller, V. M., Seegal, R. F. & Lawrence, D. A. Autoantibody-mediated neuroinflammation: pathogenesis of neuropsychiatric systemic lupus erythematosus in the NZM88 murine model. Brain Behav. Immun. 22, 949–959 (2008)
Dubois, E. L., Horowitz, R. E., Demopoulos, H. B. & Teplitz, R. NZB/NZW mice as a model of systemic lupus erythematosus. J. Am. Med. Assoc. 195, 285–289 (1966)
Baccala, R. et al. Anti-IFN-α/β receptor antibody treatment ameliorates disease in lupus-predisposed mice. J. Immunol. 189, 5976–5984 (2012)
Sakic´, B. et al. A behavioral profile of autoimmune lupus-prone MRL mice. Brain Behav. Immun. 6, 265–285 (1992)
Goldmann, T. et al. USP18 lack in microglia causes destructive interferonopathy of the mouse brain. EMBO J. 34, 1612–1629 (2015)
Crow, Y. J. et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutières syndrome at the AGS1 locus. Nat. Genet. 38, 917–920 (2006)
Meuwissen, M. E. et al. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J. Exp. Med. 213, 1163–1174 (2016)
Taylor, J. M. et al. Type-1 interferon signaling mediates neuro-inflammatory events in models of Alzheimer’s disease. Neurobiol. Aging 35, 1012–1023 (2014)
Baruch, K. et al. Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346, 89–93 (2014)
Kirou, K. A. et al. Coordinate overexpression of interferon-α-induced genes in systemic lupus erythematosus. Arthritis Rheum. 50, 3958–3967 (2004)
Trysberg, E., Carlsten, H. & Tarkowski, A. Intrathecal cytokines in systemic lupus erythematosus with central nervous system involvement. Lupus 9, 498–503 (2000)
Santer, D. M., Yoshio, T., Minota, S., Möller, T. & Elkon, K. B. Potent induction of IFN-α and chemokines by autoantibodies in the cerebrospinal fluid of patients with neuropsychiatric lupus. J. Immunol. 182, 1192–1201 (2009)
Hickman, S. E. et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16, 1896–1905 (2013)
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003)
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012)
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016)
Pan, W., Banks, W. A. & Kastin, A. J. Permeability of the blood-brain and blood-spinal cord barriers to interferons. J. Neuroimmunol. 76, 105–111 (1997)
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007)
Islam, S. et al. Quantitative single-cell RNA-seq with unique molecular identifiers. Nat. Methods 11, 163–166 (2014)
Young, M. D., Wakefield, M. J., Smyth, G. K. & Oshlack, A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11, R14 (2010)
Chatterjee, P. et al. Complement C4 maintains peripheral B-cell tolerance in a myeloid cell dependent manner. Eur. J. Immunol. 43, 2441–2450 (2013)
Tucker, K. L., Meyer, M. & Barde, Y.-A. Neurotrophins are required for nerve growth during development. Nat. Neurosci. 4, 29–37 (2001)
Armulik, A. et al. Pericytes regulate the blood–brain barrier. Nature 468, 557–561 (2010)
Ben-Zvi, A. et al. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 509, 507–511 (2014)
Schafer, D. P., Lehrman, E. K., Heller, C. T. & Stevens, B. An engulfment assay: a protocol to assess interactions between CNS phagocytes and neurons. J. Vis. Exp. (88) e51482 (2014)
Tremblay, M.-È., Lowery, R. L. & Majewska, A. K. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8, e1000527 (2010)
Bisht, K. et al. Dark microglia: a new phenotype predominantly associated with pathological states. Glia 64, 826–839 (2016)
Mori, S. & Leblond, C. P. Identification of microglia in light and electron microscopy. J. Comp. Neurol. 135, 57–79 (1969)
Acknowledgements
We thank members of the M.C.C. and G.C.T. laboratories for helpful comments on experimental design and feedback on the manuscript; M. Tsokos, B. Volpe, P. Crow, B. Stevens and S. McCarroll for helpful discussions; E. M. Carroll for assistance and L. Prince-Wright for technical support; the HMS Rodent Histopathology core, and M. Ericsson and E. Benecchi from the HMS Electron Microscopy core; L. Konnikova and S. Snapper for advice and assistance with RNAscope; B. Caldarone at the Harvard NeuroDiscovery Center’s NeuroBehavior Laboratory Core for guidance; the Boston Children’s Hospital PCMM Optical Microscopy and flow cytometry cores and the Whitehead Institute Genome Technology Core; K. Holton from the HMS Bioinformatics core and S. Bradley for assistance with RNA-seq analysis, and C. Usher for help with editing the manuscript. This research was supported by the Alliance for Lupus Research (A.L.R.—332527); NIH (AI039246) and MedImmune LLC (M.C.C.), and by the NIH (AI42269) (G.C.T.). A.R.B. was supported by a NIH T32 training grant (AI74549) and the Jeffrey Modell Foundation.
Author information
Authors and Affiliations
Contributions
A.R.B. designed and performed experiments and wrote the manuscript. J.P. assisted with flow cytometry and manuscript editing. A.D. assisted with bone marrow chimaeras and anti-IFNAR treatment. C.E.v.d.P. assisted with FACS purification of microglia, RNA-seq and manuscript editing. L.M. and G.V. helped with RNA-seq data acquisition and analysis. C.M. acquired samples and performed immunohistochemistry on tissue from patients with SLE. R.H. provided anti-IFNAR and isotype-control antibodies and advised on anti-IFNAR treatment. P.H.L. and G.C.T. provided MRL-lpr and control tissue and helpful feedback on the manuscript. M.C.C. advised on experimental design and assisted in manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
R.H. is an employee of MedImmune LLC. All other authors have no competing financial interests to declare.
Additional information
Reviewer Information Nature thanks D. Hunt, R. Klein and M. Prinz for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
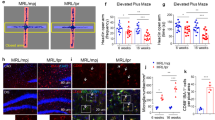
Extended Data Figure 1 Behavioural phenotyping of 564Igi mice.
a, b, 564Igi mice exhibit anxiety-like behaviours. n = 10 mice per group, *P < 0.05, unpaired t-test. c, Impaired cognitive performance of 564Igi mice. n = 10 mice per group, **P = 0.007, two-way ANOVA with Sidak’s test. d, 564Igi mice exhibit impaired spatial learning in a water T maze. n = 10 mice per group, P = 0.0001, comparison of nonlinear fits test. e, Increased social dominance/aggression was observed in 564Igi mice. n = 10 mice per group, *P = 0.04, paired t-test. f, Impaired social interaction in 564Igi mice. n = 10 mice per group, ****P < 0.0001, two-way ANOVA with Sidak’s test. g, Increased prepulse inhibition in 564Igi mice. n = 10 mice per group, *P = 0.03, ****P < 0.0001, two-way ANOVA with Sidak’s test. h, 564Igi mice have normal levels of activity and motor function in the open field test. n = 10 mice per group; P > 0.05 (not significant), one-way ANOVA with Tukey’s test. i, Rotarod performance demonstrates normal motor coordination. n = 10 mice per group; P > 0.05 (not significant), two-way ANOVA with Sidak’s test. j, Marble-burying behaviour is normal in 564Igi mice. n = 10 mice per group; P > 0.05 (not significant), unpaired t-test. k, l, Mice do not exhibit depression-like phenotypes. n = 10 mice per group; P > 0.05 (not significant), unpaired t-test. Data are mean ± s.e.m. (a–l).
Extended Data Figure 2 No significant cellular infiltration or microglia proliferation in 564Igi mice and comparison with MRL-lpr mice.
a, Representative images of haematoxylin and eosin staining show no evidence of cellular infiltration or gross anatomical abnormalities in 564Igi mice. Scale bar, 100 μm. b, Immunohistochemistry analysis shows no infiltration of B cells or T cells in the frontal cortex (top) or hippocampus (bottom). Scale bar, 20 μm. c, Representative staining in spleen for T cells (CD3), B cells (B220) and all bone-marrow-derived cells (CD45) shows antibody specificity despite lack of signal in the brain. Scale bar, 20 μm. d, Representative images of Iba1 and CD68 staining show the common microglia activation state scores observed in adult mouse tissue. Microglia, which fell into the category of 3 or higher, were pooled to form the reactive microglia population. Scale bar, 20 μm. c, Microglia density for cortex, hippocampus and cerebellum revealed similar microglia density in wild-type, 564Igi and NZB/NZW mice. d, MRL-lpr mice exhibit increased reactive microglia at 8 weeks, similar to NZB/NZW mice. n = 3 mice per group, ***P = 0.0002, unpaired t-test. Scale bar, 20 μm. Data are mean ± s.e.m. (e, f).
Extended Data Figure 3 Ifnb is not expressed and anti-IFNAR treatment is effective in both 564Igi and NZB/NZW mice.
a, qPCR for expression of Ifnb in the spleen showed similar expression levels in wild-type, 564Igi and NZB/NZW mice. n = 3 mice per group, P > 0.05, ANOVA. b, qPCR for expression of Ifnb in the hippocampus showed similar expression levels in wild-type, 564Igi and NZB/NZW mice. n = 3 mice per group, P > 0.05, ANOVA. c, qPCR for expression of Ifi44 in the spleen showed a significant increase in expression in 564Igi and NZB/NZW mice compared to wild-type mice that was reduced with anti-IFNAR treatment. n = 3 mice per group, *P < 0.049, **P = 0.002, two-way ANOVA with Sidak’s test. d, qPCR for expression of Oas1 in the spleen showed a significant increase in expression in 564Igi and NZB/W mice compared to wild-type mice that was reduced with anti-IFNAR treatment. n = 3 mice per group, *P = 0.03, ****P < 0.0001, two-way ANOVA with Tukey’s test. e, qPCR for expression of Ifi44 in the hippocampus showed a significant increase in expression in 564Igi and NZB/NZW mice compared to wild-type mice that was reduced with anti-IFNAR treatment. n = 3 mice per group, *P < 0.04, **P = 0.001, two-way ANOVA with Tukey’s test. f, qPCR for expression of Oas1 in the hippocampus showed a significant increase in expression in 564Igi and NZB/NZW mice compared to wild-type mice that was reduced with anti-IFNAR treatment. n = 3 mice per group, *P = 0.04, **P = 0.001, two-way ANOVA with Tukey’s test. g, qPCR for expression of Ifna in the spleen in 564Igi and NZB/NZW mice ± anti-IFNAR treatment. n = 3 mice per group, **P < 0.01, two-way ANOVA with Tukey’s test. h, Flow cytometry analysis of autoreactive B cell frequencies (percentage of B220+ cells) showed significant decreases after anti-IFNAR treatment. n = 4 mice per group, **P < 0.01, two-way ANOVA with Tukey’s test. i, qPCR for expression of Ifna in the hippocampus in 564Igi and NZB/NZW mice ± anti-IFNAR treatment. n = 3 mice per group, P > 0.05, two-way ANOVA with Tukey’s test. j, Representative image from a Mx1-CreTdtomato reporter mouse immunostained against the neuronal marker NeuN (red), showing clusters of neurons and that are positive for Mx1 (pseudocoloured green). Scale bar, 50 μm. Data are mean ± s.e.m. (a–i).
Extended Data Figure 4 Bone marrow chimaera approach reveals a direct role of IFNAR signalling in the brain.
a, Bone marrow extracted from wild-type (CD45.1) or 564Igi mice was administered to wild-type or Ifnar1−/− mice after lethal irradiation. Head shielding was added to protect the CNS during lethal irradiation. Mice were analysed six weeks after bone marrow transplant. b, A similar percentage of chimaerism was achieved for both Ifnar1−/− and wild-type mice. n = 4 mice per group, P = 0.9670 (not significant), unpaired t-test. c, qPCR for expression of Ifna in purified microglia revealed similar expression levels in wild-type and Ifnar1−/− recipients of wild-type or 564Igi bone marrow. n = 3 mice per group, P > 0.05, two-way ANOVA with Sidak’s test. d, qPCR for expression of Ifnb in purified microglia revealed significant upregulation in wild-type recipients of 564Igi bone marrow relative to Ifnar1−/− recipients. n = 3 mice per group, ***P = 0.0003, two-way ANOVA with Sidak’s test. Data are mean ± s.e.m. (b–d).
Extended Data Figure 5 Hippocampal brain sections from patients with SLE show increased MXA signal.
a, Relevant clinical data for the patient brain sections analysed by immunohistochemistry for MXA. Cases highlighted in yellow showed higher MXA levels than controls. b, Representative images for the cases that showed increased MXA signal. Scale bars, 100 μm. c, Antibody specificity validation: staining with an isotype control antibody (top) or only the secondary antibody (bottom) showed no signal. d, RNAscope in situ hybridization for the neuronal marker, ENO2. Quantification showed no significant differences between control and SLE cases. Data are mean ± s.e.m. n = 3 cases per group, P > 0.05, unpaired t-test.
Extended Data Figure 6 Sorting of microglia for RNA-seq analysis.
a, Macrophages were sorted on the basis of CD45high expression. b, Similar macrophage frequencies were observed in wild-type and 564Igi brain suspensions. n = 4 mice per group, P > 0.05 (not significant), unpaired t-test. c, Validation of microglia purity by post-sort flow cytometry revealed surface expression of two additional microglial markers, CD39 and CX3CR1, in >99% of sorted cells. d, RNA-seq analysis showed elevated expression of 20 out of 25 microglia-specific genes in sorted microglia relative to sorted meningeal macrophages. Data are mean ± s.e.m. (b, d).
Extended Data Figure 7 RNA-seq and Gene Ontology analysis.
a, Multidimensional scaling analysis shows clustering of samples by treatment group. Dim, dimension; FC, fold change. b, Smear plot showing significant changes in gene expression (red) in 564Igi versus wild-type mice. CPM, counts per million reads. c, Gene Ontology analysis using GOseq identified enriched pathways in microglia derived from 564Igi relative to wild-type mice. q < 0.05 for all pathways, size of the square is relative to q value. d, Distribution of genes classified as IFNAR-dependent or -independent. e, Microglia sensome genes that are significantly upregulated in 564Igi versus wild-type mice. Wild type versus 564Igi: all genes, q < 0.05; *564Igi versus 564Igi + anti-IFNAR: q < 0.05, GLM likelihood ratio test. Data are mean ± s.e.m.
Extended Data Figure 8 Interferon can enter via an intact blood–brain barrier and is sufficient to trigger engulfment of neuronal material by microglia.
a, Gating for tau-GFP+ microglia for engulfment analysis based on tau-GFP− control mice (gate set to <0.5% in tau-GFP− mice). b, The tau-GFP mean fluorescence intensity (MFI) for microglia was significantly higher in the frontal cortex of 564Igi relative to wild-type mice. n = 3 mice per group, ****P < 0.0001, two-way ANOVA with Sidak’s test. c, Intravenous injection of 10 kDa FITC–dextran tracer revealed no leakage of the dye from blood vessels (CD31+) in 564Igi mice up to 24 weeks of age. d, Staining for IgG revealed no increase in IgG deposition within the CNS in 564Igi mice compared to C57BL/6 controls, whereas MRL-lpr did show a notable increase. e, Colocalization of FITC–dextran with CD31 (blood vessels) showed that similar colocalization was observed in 564Igi mice and controls, indicating that similar amounts of dye were contained within vessels in both groups. n = 3 mice per group, P > 0.05, unpaired t-test. f, Quantification of MFI for IgG revealed no significant changes between 564Igi and C57BL/6 controls. MRL-lpr mice showed a significant increase compared to MRL-mpj controls. n = 3 mice per group, ****P < 0.0001, one-way ANOVA with Tukey’s test. g, h, Flow cytometry analysis of tau-GFP mice injected with IFNα (g) or IFNβ (h) demonstrated significant increases in tau-GFP+ frequencies compared to vehicle. n = 3 mice per group, *P < 0.05, **P < 0.01, unpaired t-test. Data are mean ± s.e.m. (b, e–h).
Extended Data Figure 9 Validation of microglia engulfment data.
a, Left, staining of Iba1 revealed inclusions within microglia cytoplasm (white arrows and inset) and lysosomes (white asterisk). Right, some inclusions contained structures consistent with synaptic vesicles (white arrows, right inset). b, Permeabilized microglia were stained in parallel with similar concentrations of Alexa-633 labelled mouse IgG1. Fluorescence was several logs lower than observed with SV2 staining. c, To verify internalization of SV2, staining was also done on non-permeabilized microglia. Very few (0.78%) microglia showed signal over the isotype control. No microglia could be detected in the SV2high gate used for analysis in Fig. 4. d, To further validate internalization of SV2, permeabilized microglia were slide-mounted by cytospin and imaged at 63× by confocal. SV2 signal appeared colocalized with CD68 in many, but not all, microglia.
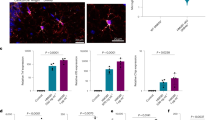
Extended Data Figure 10 Synapse loss, but not neuron or axon loss, is observed in the frontal cortex of 564Igi mice.
a, Staining for presynaptic (synaptophysin, red) and postsynaptic (homer, green) markers revealed structural synapses (colocalized markers, yellow). Scale bar, 25 μm. b, c, Significant decreases were seen in synaptophysin (b) and homer (c) puncta. n = 3 mice per group, *P < 0.05, **P < 0.01, unpaired t-test. d, Reduced synapse density was also observed in 8-week-old MRL-lpr mice by immunohistochemistry. n = 3 mice per group, *P < 0.05, unpaired t-test. e, Staining for the neuronal marker NeuN revealed similar neuronal density in the frontal cortex of 564Igi and wild-type mice. n = 3 mice per group, P = 0.85 (not significant), unpaired t-test. f, Staining for the axonal marker neurofilament-H showed a slight but not significant decrease in 564Igi axon density. n = 3 mice per group, P > 0.05 (not significant), unpaired t-test. Data are mean ± s.e.m. (b–f).
Rights and permissions
About this article
Cite this article
Bialas, A., Presumey, J., Das, A. et al. Microglia-dependent synapse loss in type I interferon-mediated lupus. Nature 546, 539–543 (2017). https://doi.org/10.1038/nature22821
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22821
This article is cited by
-
Exosome Content–Mediated Signaling Pathways in Multiple Sclerosis
Molecular Neurobiology (2024)
-
Novel CH25H+ and OASL+ microglia subclusters play distinct roles in cerebral ischemic stroke
Journal of Neuroinflammation (2023)
-
Synaptic loss in a mouse model of euthyroid Hashimoto’s thyroiditis: possible involvement of the microglia
BMC Neuroscience (2022)
-
Pyruvate kinase isoform M2 impairs cognition in systemic lupus erythematosus by promoting microglial synaptic pruning via the β-catenin signaling pathway
Journal of Neuroinflammation (2021)
-
Human stem cell models to study host–virus interactions in the central nervous system
Nature Reviews Immunology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.