Abstract
Sex differences in physiology and disease susceptibility are commonly attributed to developmental and/or hormonal factors, but there is increasing realization that cell-intrinsic mechanisms play important and persistent roles1,2. Here we use the Drosophila melanogaster intestine to investigate the nature and importance of cellular sex in an adult somatic organ in vivo. We find that the adult intestinal epithelium is a cellular mosaic of different sex differentiation pathways, and displays extensive sex differences in expression of genes with roles in growth and metabolism. Cell-specific reversals of the sexual identity of adult intestinal stem cells uncovers the key role this identity has in controlling organ size, reproductive plasticity and response to genetically induced tumours. Unlike previous examples of sexually dimorphic somatic stem cell activity, the sex differences in intestinal stem cell behaviour arise from intrinsic mechanisms that control cell cycle duration and involve a new doublesex- and fruitless-independent branch of the sex differentiation pathway downstream of transformer. Together, our findings indicate that the plasticity of an adult somatic organ is reversibly controlled by its sexual identity, imparted by a new mechanism that may be active in more tissues than previously recognized.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Arnold, A. P. The end of gonad-centric sex determination in mammals. Trends Genet. 28, 55–61 (2012)
Ober, C., Loisel, D. A. & Gilad, Y. Sex-specific genetic architecture of human disease. Nature Rev. Genet. 9, 911–922 (2008)
Cognigni, P., Bailey, A. P. & Miguel-Aliaga, I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 13, 92–104 (2011)
Lucchesi, J. C. & Kuroda, M. I. Dosage compensation in Drosophila. Cold Spring Harb. Perspect. Biol. 7, http://dx.doi.org/10.1101/cshperspect.a019398 (2015)
Boggs, R. T., Gregor, P., Idriss, S., Belote, J. M. & McKeown, M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell 50, 739–747 (1987)
Camara, N., Whitworth, C. & Van Doren, M. The creation of sexual dimorphism in the Drosophila soma. Curr. Top. Dev. Biol. 83, 65–107 (2008)
Christiansen, A. E., Keisman, E. L., Ahmad, S. M. & Baker, B. S. Sex comes in from the cold: the integration of sex and pattern. Trends Genet. 18, 510–516 (2002)
Villella, A. & Hall, J. C. Neurogenetics of courtship and mating in Drosophila. Adv. Genet. 62, 67–184 (2008)
Amcheslavsky, A., Jiang, J. & Ip, Y. T. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 4, 49–61 (2009)
Arthur, B. I. Jr, Jallon, J. M., Caflisch, B., Choffat, Y. & Nothiger, R. Sexual behaviour in Drosophila is irreversibly programmed during a critical period. Current Biol. 8, 1187–1190 (1998)
Ferveur, J. F. et al. Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science 276, 1555–1558 (1997)
Clough, E. et al. Sex- and tissue-specific functions of Drosophila doublesex transcription factor target genes. Dev. Cell 31, 761–773 (2014)
Ma, Q., Wawersik, M. & Matunis, E. L. The Jak–STAT target Chinmo prevents sex transformation of adult stem cells in the Drosophila testis niche. Dev. Cell 31, 474–486 (2014)
Matson, C. K. et al. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476, 101–104 (2011)
Shapiro-Kulnane, L., Smolko, A. E. & Salz, H. K. Maintenance of Drosophila germline stem cell sexual identity in oogenesis and tumorigenesis. Development 142, 1073–1082 (2015)
Uhlenhaut, N. H. et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139, 1130–1142 (2009)
Caldwell, J. C., Fineberg, S. K. & Eberl, D. F. reduced ocelli encodes the leucine rich repeat protein Pray For Elves in Drosophila melanogaster. Fly 1, 146–152 (2007)
Handke, B. et al. The hemolymph proteome of fed and starved Drosophila larvae. PLoS ONE 8, e67208 (2013)
Kawamura, K., Shibata, T., Saget, O., Peel, D. & Bryant, P. J. A new family of growth factors produced by the fat body and active on Drosophila imaginal disc cells. Development 126, 211–219 (1999)
Brown, J. B. et al. Diversity and dynamics of the Drosophila transcriptome. Nature 512, 393–399 (2014)
Reiff, T. et al. Endocrine remodelling of the adult intestine sustains reproduction in Drosophila. eLife 4, e06930 (2015)
Ohlstein, B. & Spradling, A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470–474 (2006)
Patel, P. H. & Edgar, B. A. Tissue design: how Drosophila tumors remodel their neighborhood. Semin. Cell Dev. Biol. 28, 86–95 (2014)
Evans, D. S. & Cline, T. W. Drosophila switch gene Sex-lethal can bypass its switch-gene target transformer to regulate aspects of female behavior. Proc. Natl Acad. Sci. USA 110, E4474–E4481 (2013)
Finley, K. D., Taylor, B. J., Milstein, M. & McKeown, M. dissatisfaction, a gene involved in sex-specific behavior and neural development of Drosophila melanogaster. Proc. Natl Acad. Sci. USA 94, 913–918 (1997)
Robinett, C. C., Vaughan, A. G., Knapp, J. M. & Baker, B. S. Sex and the single cell. II. There is a time and place for sex. PLoS Biol. 8, e1000365 (2010)
Markle, J. G. et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088 (2013)
Steegenga, W. T. et al. Sexually dimorphic characteristics of the small intestine and colon of prepubescent C57BL/6 mice. Biol. Sex Diff. 5, 11 (2014)
Cook, M. B. et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol. Biomarkers Prev. 18, 1174–1182 (2009)
Siudeja, K. et al. Frequent somatic mutation in adult intestinal stem cells drives neoplasia and genetic mosaicism during aging. Cell Stem Cell 17, 663–674 (2015)
Ruiz, M. F., Sarno, F., Zorrilla, S., Rivas, G. & Sanchez, L. Biochemical and functional analysis of Drosophila-sciara chimeric sex-lethal proteins. PLoS ONE 8, e65171 (2013)
Lu, B., Ackerman, L., Jan, L. Y. & Jan, Y. N. Modes of protein movement that lead to the asymmetric localization of partner of Numb during Drosophila neuroblast division. Mol. Cell 4, 883–891 (1999)
Zielke, N. et al. Fly-FUCCI: A versatile tool for studying cell proliferation in complex tissues. Cell Rep. 7, 588–598 (2014)
Ferveur, J. F., Stortkuhl, K. F., Stocker, R. F. & Greenspan, R. J. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science 267, 902–905 (1995)
Lee, G., Hall, J. C. & Park, J. H. Doublesex gene expression in the central nervous system of Drosophila melanogaster. J. Neurogenet. 16, 229–248 (2002)
Merrill, C. E., Sherertz, T. M., Walker, W. B. & Zwiebel, L. J. Odorant-specific requirements for arrestin function in Drosophila olfaction. J. Neurobiol. 63, 15–28 (2005)
Daborn, P. J. et al. Evaluating the insecticide resistance potential of eight Drosophila melanogaster cytochrome P450 genes by transgenic over-expression. Insect Biochem. Mol. Biol. 37, 512–519 (2007)
Bardin, A. J., Perdigoto, C. N., Southall, T. D., Brand, A. H. & Schweisguth, F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development 137, 705–714 (2010)
de Celis, J. F. & Bray, S. Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development 124, 3241–3251 (1997)
Schweitzer, R., Shaharabany, M., Seger, R. & Shilo, B. Z. Secreted Spitz triggers the DER signaling pathway and is a limiting component in embryonic ventral ectoderm determination. Genes Dev. 9, 1518–1529 (1995)
Martorell, Ò. et al. Conserved mechanisms of tumorigenesis in the Drosophila adult midgut. PLoS ONE 9, e88413 (2014)
Kohl, J., Ostrovsky, A. D., Frechter, S. & Jefferis, G. S. A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell 155, 1610–1623 (2013)
Mellert, D. J., Robinett, C. C. & Baker, B. S. doublesex functions early and late in gustatory sense organ development. PLoS ONE 7, e51489 (2012)
Cande, J., Stern, D. L., Morita, T., Prud’homme, B. & Gompel, N. Looking under the lamp post: neither fruitless nor doublesex has evolved to generate divergent male courtship in Drosophila. Cell Rep. 8, 363–370 (2014)
Baker, B. S., Hoff, G., Kaufman, T. C., Wolfner, M. F. & Hazelrigg, T. The doublesex locus of Drosophila melanogaster and its flanking regions: a cytogenetic analysis. Genetics 127, 125–138 (1991)
Duncan, I. W. & Kaufman, T. C. Cytogenic analysis of chromosome 3 in Drosophila melanogaster: mapping of the proximal portion of the right arm. Genetics 80, 733–752 (1975)
Demir, E. & Dickson, B. J. fruitless splicing specifies male courtship behavior in Drosophila. Cell 121, 785–794 (2005)
Mellert, D. J., Knapp, J. M., Manoli, D. S., Meissner, G. W. & Baker, B. S. Midline crossing by gustatory receptor neuron axons is regulated by fruitless, doublesex and the Roundabout receptors. Development 137, 323–332 (2010)
Belote, J. M., Hoffmann, F. M., McKeown, M., Chorsky, R. L. & Baker, B. S. Cytogenetic analysis of chromosome region 73AD of Drosophila melanogaster. Genetics 125, 783–793 (1990)
Sturtevant, A. H. A gene in Drosophila melanogaster that transforms females into males. Genetics 30, 297–299 (1945)
Mattox, W. & Baker, B. S. Autoregulation of the splicing of transcripts from the transformer-2 gene of Drosophila. Genes Dev. 5, 786–796 (1991)
Belote, J. M. & Baker, B. S. Sexual behavior: its genetic control during development and adulthood in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 84, 8026–8030 (1987)
Gowen, J. W. & Fung, S. T. C. Determination of sex through genes in a major sex locus in Drosophila melanogaster. Heredity 11, 397–402 (1957)
Anand, A. et al. Molecular genetic dissection of the sex-specific and vital functions of the Drosophila melanogaster sex determination gene fruitless. Genetics 158, 1569–1595 (2001)
Haenlin, M., Kramatschek, B. & Campos-Ortega, J. A. The pattern of transcription of the neurogenic gene Delta of Drosophila melanogaster. Development 110, 905–914 (1990)
Morel, V. & Schweisguth, F. Repression by suppressor of hairless and activation by Notch are required to define a single row of single-minded expressing cells in the Drosophila embryo. Genes Dev. 14, 377–388 (2000)
Furriols, M. & Bray, S. A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr. Biol. 11, 60–64 (2001)
Rideout, E. J., Dornan, A. J., Neville, M. C., Eadie, S. & Goodwin, S. F. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nature Neurosci. 13, 458–466 (2010)
Manoli, D. S. et al. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 436, 395–400 (2005)
Wang, L., Zeng, X., Ryoo, H. D. & Jasper, H. Integration of UPRER and oxidative stress signaling in the control of intestinal stem cell proliferation. PLoS Genet. 10, e1004568 (2014)
Zeng, X., Chauhan, C. & Hou, S. X. Characterization of midgut stem cell- and enteroblast-specific Gal4 lines in Drosophila. Genesis 48, 607–611 (2010)
Phillips, M. D. & Thomas, G. H. Brush border spectrin is required for early endosome recycling in Drosophila. J. Cell Sci. 119, 1361–1370 (2006)
Balakireva, M., Stocker, R. F., Gendre, N. & Ferveur, J. F. Voila, a new Drosophila courtship variant that affects the nervous system: behavioral, neural, and genetic characterization. J. Neurosci. 18, 4335–4343 (1998)
Pan, Y., Robinett, C. C. & Baker, B. S. Turning males on: activation of male courtship behavior in Drosophila melanogaster. PLoS ONE 6, e21144 (2011)
Shiga, Y., Tanaka-Matakatsu, M. & Hayashi, S. A nuclear GFP/β-galactosidase fusion protein as a marker for morphogenesis in living Drosophila. Dev. Growth Differ. 38, 99–106 (1996)
Misra, J. R., Horner, M. A., Lam, G. & Thummel, C. S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 25, 1796–1806 (2011)
Baena-Lopez, L. A., Alexandre, C., Mitchell, A., Pasakarnis, L. & Vincent, J. P. Accelerated homologous recombination and subsequent genome modification in Drosophila. Development 140, 4818–4825 (2013)
Bischof, J., Maeda, R. K., Hediger, M., Karch, F. & Basler, K. An optimized transgenesis system for Drosophila using germ-line-specific φC31 integrases. Proc. Natl Acad. Sci. USA 104, 3312–3317 (2007)
Kimura, K., Ote, M., Tazawa, T. & Yamamoto, D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature 438, 229–233 (2005)
Yeo, S. L. et al. On the functional overlap between two Drosophila POU homeo domain genes and the cell fate specification of a CNS neural precursor. Genes Dev. 9, 1223–1236 (1995)
Green, E. W., Fedele, G., Giorgini, F. & Kyriacou, C. P. A Drosophila RNAi collection is subject to dominant phenotypic effects. Nature Methods 11, 222–223 (2014)
R Development Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2014)
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013)
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015)
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010)
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009)
Turro, E. et al. Haplotype and isoform specific expression estimation using multi-mapping RNA-seq reads. Genome Biol. 12, R13 (2011)
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014)
Saeed, A. I. et al. TM4 microarray software suite. Methods Enzymol. 411, 134–193 (2006)
Jiang, H. & Edgar, B. A. Intestinal stem cell function in Drosophila and mice. Curr. Opin. Genet. Dev. 22, 354–360 (2012)
Lemaitre, B. & Miguel-Aliaga, I. The digestive tract of Drosophila melanogaster. Annu. Rev. Genet. 47, 377–404 (2013)
Kelley, R. L. et al. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell 81, 867–877 (1995)
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999)
Jiang, H. et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355 (2009)
Patel, P. H., Dutta, D. & Edgar, B. A. Niche appropriation by Drosophila intestinal stem cell tumours. Nature Cell Biol. 17, 1182–1192 (2015)
Buchon, N. et al. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 3, 1725–1738 (2013)
Micchelli, C. A. & Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 (2006)
Kelso, R. J. Flytrap, a database documenting a GFP protein-trap insertion screen in Drosophila melanogaster. Nucleic Acids Res. 32, D418–D420 (2004)
Spradling, A. C. et al. The Berkeley Drosophila genome project gene disruption project. Single P-element insertions mutating 25% of vital Drosophila genes. Genetics 153, 135–177 (1999)
Riabinina, O. et al. Improved and expanded Q-system reagents for genetic manipulations. Nature Methods 12, 219–222 (2015)
Luo, L., Liao, Y. J., Jan, L. Y. & Jan, Y. N. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8, 1787–1802 (1994)
Ghazi, A., Anant, S. & VijayRaghavan, K. Apterous mediates development of direct flight muscles autonomously and indirect flight muscles through epidermal cues. Development 127, 5309–5318 (2000)
Xu, T. & Rubin, G. M. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 (1993)
Golic, K. G. Site-specific recombination between homologous chromosomes in Drosophila. Science 252, 958–961 (1991)
Acknowledgements
We thank K. Vanezis and L. Game for technical assistance, and C. Gonzalez, T. Carroll and D. Perea for discussions. B. Baker, A. Bardin, A. Baena-Lopez, S. Bray, A. Casali, T. Cline, S. Cohen, D. Eberl, S. Goodwin, G. Jefferis, L. Jones, R. Niwa, B. Prud’homme, I. Salecker, L. Sanchez, C. Thummel, J. Treisman, M. Vidal, D. Yamamoto and L. Zwiebel shared reagents. D. Hadjieconomou, J. Jacobson, G. King and E. Parra-Peralbo provided comments on the manuscript. This work was funded by an ERC Starting Grant to I.M.-A. (ERCStG 310411) and MRC intramural funding. B.H. holds an EMBO long-term fellowship and I.M.-A. is a member of, and is supported by, the EMBO Young Investigator Programme.
Author information
Authors and Affiliations
Contributions
B.H. and I.M.-A. designed and conceived the study. S.K. performed statistical analyses of RNA-seq data, B.H. performed all other experiments in the study and analysed data. I.M.-A. analysed data and wrote the manuscript, with contributions from B.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Sexually dimorphic transcription and splicing in the adult midgut.
a, Number and percentage of genes with sexually dimorphic gene expression, as revealed by RNA-seq transcriptional profiling of virgin male and female dissected midguts (P < 0.05 cutoff). b, Volcano plot displaying all genes with detectable midgut expression. Female/male ratio of gene expression is shown on the x axis (in log2 scale) and significance is displayed on the y axis as the negative logarithm (log10 scale) of the adjusted P value. Genes with significantly upregulated (P < 0.05 cutoff) expression in males and females are coloured in blue and red, respectively. Other genes are displayed in black. Genes with known sex-specific transcription are displayed as red (female-enriched) or blue (male-enriched) open circles. c, d, Comparable analyses for sex-biased isoforms belonging to genes with multiple transcripts. We identifed 714 sex-biased isoforms belonging to a total of 603 genes. Isoforms resulting from known sex-specific alternative splicing are displayed as in panel b. e, Female/male ratios of overall transcript abundance (left graph) and abundance of sex-biased isoforms (right graph) for the members of the Drosophila sex determination pathway as revealed by RNA-seq analysis of the adult midgut. We note a sexual dimorphism in dsx transcript levels. f, Venn diagram illustrating the overlap between the genes showing sex-biased expression (overall transcript abundance, light grey, 1,305 genes) and sex-biased alternative splicing (sex-biased isoforms, dark grey, 603 genes) in the adult midgut. Known members of the sex determination pathway are displayed as examples. g, Heat maps displaying genes with sexually dimorphic expression clustered by enrichment in specific biological processes, as revealed by Gene Ontology enrichment analysis. Genes with sexually dimorphic expression belonging to the top 4 enriched biological processes are shown. h, i, Real-time qRT–PCR data for a subset of genes for which RNA-seq transcriptional profiling experiments revealed sexually dimorphic expression (h) or isoforms (i). RNA was obtained from midguts from virgin male and female samples (same genotypes as for the RNA-seq experiments). For each gene/isoform, expression abundance was arbitrarily set up at 100% for the sex with the highest expression level, and percentage of that expression is displayed for the other sex. See Methods for details and Supplementary Table 1 for a full list of names and quality scores, and Supplementary Information for full genotypes.
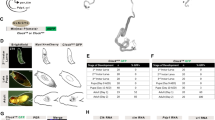
Extended Data Figure 2 Cell type-specific expression of sex determinants in the adult intestinal epithelium of virgin flies.
a, In the adult Drosophila midgut, resident stem cells (ISCs) and their postmitotic daughter cells (EBs) maintain the adult intestinal epithelium during normal homeostasis and regenerate it after injury by giving rise to two types of differentiated progeny: ECs and EECs80,81. The posterior midgut area used to visualize and quantify phenotypes is boxed. The following Gal4 drivers were used to label and/or genetically manipulate these four different cell types present in the adult intestinal epithelium: mex1 for ECs, prosv1 for EECs, esg for both ISCs and EBs, and Supressor of Hairless (Su(H)) for EBs alone. b, The canonical sex determination pathway in somatic cells of Drosophila melanogaster, consisting of a cascade of sex-specific alternative splicing events culminating in the production of sex-specific transcription factors encoded by Dsx and FruM. In females, Sex lethal (Sxl) is activated and regulates the splicing of transformer (tra) pre-mRNA, resulting in the production of TraF. TraF regulates the female-specific splicing of dsx pre-mRNA (dsxF) and fru transcript coming from the P1 promoter (fruP1, giving rise to fruM). In males, Sxl is not expressed and no functional Tra is produced, resulting in default splicing of dsx and fru pre-mRNAs, leading to FruM and DsxM proteins, respectively. The resulting male- and female-specific Dsx and Fru isoforms confer sexual identity to the cells in which they are produced. In addition, in females, Sxl represses dosage compensation by inhibiting Msl-2 expression. The tables summarize the cell-specific expression profiles of the sex determinants in adult midguts of virgin males and females, shown in the panels below. c, Sxl protein (in red) is expressed only in female midguts. Co-staining with ISC/EB reporters indicates that Sxl is found esg-positive progenitors (ISCs: GFP-positive and LacZ-negative cells, and EBs: GFP-positive and LacZ-positive cells). It is also expressed in female polyploid ECs (GFP- (in green) and LacZ- (in blue) negative cells). Co-staining with prosV1 reporter indicates that it is also expressed in EECs. d, Msl-2 protein is found in the same cell types only in males (staining is confined to the X chromosome, consistent with the signal observed in non-intestinal tissues82). e, A new reporter of tra promoter activity (tra-LacZ, see Methods for details) is broadly expressed in the epithelium of both male and female midguts, including ISCs and EBs (as revealed by co-staining with esg-Gal4-driven GFP) and ECs (GFP-negative cells with large nuclei). Co-staining with prosV1 reporter indicates that it is also expressed in EECs. f, A dsx-Gal4 reporter (visualized with a GFP reporter that has been false-coloured in red for consistency with the other panels) is active in male and female polyploid ECs (LacZ-negative cells), and is inactive in esg-positive progenitors (LacZ-positive cells, in green). Dsx protein (visualized using a DsxDBD-specific antibody in red) is expressed strongly only in males bot not females, indicating that the sexual dimorphism in dsx transcript levels found in our RNA-seq analyses (Extended Data Fig. 1e) is further enhanced at the protein level. Co-staining of the same antibody with the EC marker mex1-Gal4 confirms expression in ECs. Cytoplasmic dsx-Gal4-driven expression of an mTomato reporter is apparent in ECs (visualized as large cells by co-staining with the membrane-enriched marker Armadillo), but is absent from EECs (as revealed by the gaps in mTomato expression in cells that are labelled with Pros. g, The fruP1-Gal4 reporter (which labels the only sexually dimorphic fru transcript that gives rise to FruM protein) is inactive in both male and female midguts, as revealed by lack of GFP signal (false-coloured in red for consistency with other panels). Consistent with the lack of fruP1-Gal4 expression, a FruM-specific antibody (in red) is not expressed in the male midgut. An independent dsx reporter (dsxΔ2-Gal4) is expressed in polyploid ECs, consistent with the data displayed in f. See Supplementary Information for full genotypes.
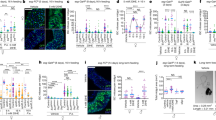
Extended Data Figure 3 Sxl controls intrinsic sex differences in adult ISC proliferation independently of dosage compensation.
a, Immunocytochemistry using a Sxl-specific antibody indicates that adult-restricted downregulation of Sxl in intestinal progenitors (ISCs/EBs)—achieved by Gal80TS-controlled expression of a Sxl RNAi transgene—efficiently downregulates Sxl expression in progenitors, but not large polyploid ECs. Conversely, efficient ectopic Sxl protein expression is obtained by expression of a UAS-Sxl transgene in adult ISCs/EBs of male flies. In all panels Sxl antibody is in red; DNA: DAPI, in blue; ISC/EB marker: GFP, in green. b, Quantifications of the number of cells inside control MARCM83 clones, or MARCM clones expressing RNAi transgenes directed against Sxl following DSS treatment. 5 days after clone induction by heat shock, female clones are larger than male clones only when Sxl is present, confirming the cell autonomy of Sxl action. c, Additional controls for Fig. 1a, and confirmation of phenotypes using an independent RNAi transgene. This second RNAi transgene against Sxl (different from the one used in Fig. 1a) reduces the number of pH3-positive cells in DSS-treated female midguts when expressed from esgTS in adults ISCs/EBs, confirming an adult progenitor-specific requirement for Sxl in promoting damage-induced cell divisions in female flies. d, Adult-specific downregulation of Sxl in adult visceral muscle (using the vm driver), trachea (btl-Gal4 and DSRF-Gal4), neurons (nSyb-Gal4, Elav-Gal4), or fat body (stripe-Gal4) does not reduce DSS-induced ISC proliferation in females, By contrast, Sxl downregulation using an ISC/EB driver with suppressed neuronal expression (esg-Gal4 combined with nSyb-Gal80) effectively reduces DSS-induced ISC proliferation in females. Together, these results indicate that Sxl does not control sexually dimorphic DSS-induced ISCs proliferation from non-ISC cells. e, MARCM clones expressing a third RNAi transgene against Sxl (distinct from those used in Fig. 1 and above) are smaller than control clones in females, whereas their size is comparable to that of wild-type or Sxl-RNAi clones in males. This confirms that, during normal homeostasis, female ISCs divide more often than male ISCs because of the cell-autonomous action of Sxl. The graph shows quantifications of the number of cells within each clone 15 days after clone induction by heat shock, and the confocal images show representative clones (labelled in green with GFP) for each genotype. f, Clonal analyses of homeostatic proliferation using the inducible esg flip-out system (which labels progenitors and their progeny generated within a defined temporal window84) in midguts of control males, control females and females in which Sxl downregulation has been confined to adult progenitors. 15 days after induction, the size (assessed as the percentage of GFP-positive area) of control female clones was significantly larger than that of male clones, but both became comparable upon adult-specific Sxl downregulation using Sxl RNAi transgenes. The graph shows area quantifications for each sex/genotype, and the confocal images show representative clones for each genotype. g, Immunohistochemical detection of histone H4 lysine 16 (H4Lys16) acetylation (in red) indicates that adult-specific downregulation of msl-2 in male intestinal progenitors (ISCs/EBs marker: GFP, in green) results in loss of H4Lys16 acetylation of the X chromosome. h, Efficient Msl-2 misexpression in adult female intestinal progenitors (ISCs/EBs marker: GFP, in green) is confirmed by immunocytochemistry using an HA-specific antibody (in red). n denotes the number of guts (c, d, f) or clones (b, e) that were analysed for each genotype. Results were combined from at least two independent experiments. See Supplementary Information for full genotypes.
Extended Data Figure 4 Sexually dimorphic proliferation does not result from sex differences in differentiation.
a, Markers for all four intestinal cell types are still apparent following adult-specific downregulation of Sxl in the intestinal progenitors of females—achieved by Gal80TS-controlled expression of a Sxl RNAi transgene. Indeed, expression of esg-Gal4 (ISC/EBs), Su(H)-LacZ (EBs), Pdm1 (ECs) and Pros (EECs) can be readily detected, suggesting that Sxl downregulation in females (which results in reduced ISC proliferation) does not have a major effect on differentiation. Sxl staining confirms efficient downregulation in ISCs/EBs, but not neighbouring cells. b, The same markers reveal that the differentiation defect resulting from Notch downregulation, previously reported in females, is also apparent in males (note loss of Su(H)-LacZ following Notch downregulation in both males and females), suggesting that sex differences in differentiation do not contribute to the sex differences in susceptibility to Notch-induced tumours. Co-expression of a mitogen (secreted Spitz, sSpi) abrogates the sex differences in tumour susceptibility by efficiently triggering hyperplasia also in males, as revealed by an expanded progenitor (GFP-positive) area in both males and females. The identity of these tumours in males is also comparable to that previously show for Notch tumours in females (consisting of high Pros-positive EEC-like cells and low Pros-positive neoplastic ISC-like cells85. This further suggests that the sex differences in Notch-induced tumour susceptibility do not arise from sexually dimorphic differentiation effects, but result from sex differences in ISC proliferation. See Supplementary Information for full genotypes.
Extended Data Figure 5 tra, but not tra2, controls intrinsic sex differences in adult ISC proliferation.
a, Additional controls for Fig. 2a, and confirmation of phenotypes using independent RNAi transgenes. Two additional RNAi transgenes against tra reduce the number of pH3-positive cells in DSS-treated female, but not male, midguts when expressed from esgTS in adults ISCs/EBs, confirming an adult progenitor-specific requirement for tra in promoting damage-induced cell divisions in female flies. b, tra1 MARCM mutant clones are smaller than control clones in females, whereas their size is comparable to that of wild-type or tra1 clones in males. This confirms that female ISCs divide more often than male ISCs because of the cell-autonomous action of tra. The graph shows quantifications of clone size (in arbitrary units of GFP fluorescence, as described in Methods) 15 days after clone induction by heat shock, and the confocal images show representative clones (labelled in green with GFP) for each genotype. c, Ubiquitous, adult-restricted traF expression from tub-Gal80TS in males increases the number of pH3-positive cells following DSS treatment to levels comparable to those of female flies. d, Re-introduction of this traF transgene specifically in adult ISCs/EBs rescues the reduced, male-like intestinal proliferation (as assessed by the number of pH3-positive cells) of tra null mutant females entirely lacking the tra gene from all their tissues (traKO/tra1) to levels comparable to those of control females. Expression of this transgene in control heterozygous female flies (traKO/+ esgTS>traF) does not significantly increase their proliferation (in fact, it reduces it slightly relative to traKO/+ esgTS> controls, likely as a consequence of its overexpression). e, Clonal analyses of homeostatic proliferation using the inducible esg flip-out system (which labels progenitors and their progeny generated within a defined temporal window84) in midguts of control females and females in which tra downregulation has been confined to adult progenitors. 15 days after induction, the size (assessed as the percentage of GFP-positive area) of control clones is significantly larger than that of tra-RNAi clones. The graph shows area quantifications for each genotype, and the confocal images show representative clones for each genotype. f, Consistent with the tra mutant clonal analysis in Fig. 2c, quantifications of clone size (number of cells per clone) reveal that MARCM clones in which tra has been downregulated are smaller than control clones only in females. Their size is comparable to that of wild-type or tra-downregulated mutant clones in males. The confocal images show representative clones (labelled in green with GFP) for each genotype in females. g, qRT–PCR quantifications of relative abundance of traF, dsxF, dsxM and Yp1 transcripts in adult-specific tra2 mutants (tra2B/ts1 grown at permissive temperatures, then switched to the restrictive temperature 4 days after eclosion and transcriptionally profiled following 10 additional days at the restrictive temperature) and controls (tra2B/+). In tra2 mutant females, dsxF is lost, dsxM is upregulated to levels comparable to those of control males and Yp1 (a DsxF target) is lost (to levels also comparable to those of males). tra mutants (traDf(3L)st-j7/KO) were also used as a positive control. n denotes the number of guts (a, c, d, e) or clones (b, f) that were analysed for each genotype. Results were combined from at least two independent experiments. See Supplementary Information for full genotypes.
Extended Data Figure 6 dsx- and fruM-independent control of sexually dimorphic proliferation in adult intestinal stem cells.
a, Adult-restricted downregulation of dsx (achieved by co-expression of a dsx-RNAi transgene and Dicer-2 (Dcr-2) in ISCs/EBs) has no effect on the compensatory ISC proliferation observed upon DSS treatment in either males or females. dsxF expression in the same conditions does not increase ISC proliferation in either males or females. b, dsxF expression does not rescue the reduced proliferation resulting from tra downregulation in males. Representative images for each genotype are shown in both a and b (DNA: DAPI, in blue; ISC/EB marker: GFP, in green). c, The size of dsx null mutant (dsx1) MARCM clones (quantified in arbitrary units of GFP fluorescence as described in Methods) is comparable to that of controls in both sexes 15 days after clone induction by heat shock. Confocal images show representative clones (labelled in green with GFP) for each genotype. d, Quantifications of clone size in control and dsx null mutant (dsx1) MARCM clones in the midguts of DSS-treated males and females. 5 days after clone induction by heat shock, there are no significant differences in clone size (quantified in arbitrary units of GFP fluorescence as described in Methods) between control and mutants clones in either males or females. Confocal images show representative clones (labelled in green with GFP) for each genotype. e, An RNAi transgene against fru does not reduce the number of pH3-positive cells in DSS-treated midguts when expressed from esgTS in the adult ISCs/EBs of either males or females. Confocal images show that number of intestinal progenitors (esg-positive cells in green) is also unaffected by this manipulation. f, Quantifications of the number of pH3-positive cells upon DSS treatment indicates that the sexual dimorphism in ISC proliferation is unaffected in females with forced fruM expression (fruM/fru4–40) or in males with forced fruF expression (fruF/fru4–40). g, ISC proliferation is unaffected in the migduts of DSS-treated males and females entirely lacking dsx (dsx∆/dsx1), or producing only DsxF (dsx∆/dsx11) or DsxM (dsx∆/dsxD). ISC proliferation is also unaffected in dsx, fruM double null mutant males and females (dsx∆,Df(3R)Exel6179/dsx1, fruP1.LexA), and in dsx null mutants in which fruM is ectopically produced in females (dsx∆,Df(3R)Exel6179/dsx1, fru∆tra). n denotes the number of guts (a, b, e, f, g) or clones (c, d) that were analysed for each genotype. Results were combined from at least two independent experiments. See Supplementary Information for full genotypes.
Extended Data Figure 7 tra targets in adult ISCs.
a, Scatter plot of all 1,346 genes with tra-dependent expression in the adult fly midgut. For each gene, control female/tra null mutant female (Df(3L)st-j7/traKO) fold differences in transcript abundance (x axis, log2 scale) are plotted against tra mutant female with feminized ISCs (adult-restricted rescue of traF in ISCs/EBs)/tra mutant female fold differences (y axis, log2 scale). Genes with tra-sensitive expression and significantly repressed by traF in ISC/EBs (P < 0.05 cutoff) are therefore found in the left-bottom quadrant and are displayed in blue, whereas those significantly activated by traF are found in the top-right quadrant and are displayed in red. Genes with tra-dependent transcription, independent of the action of traF in intestinal precursors are displayed in black. b, Comparable analysis of tra-dependent alternative splicing. c, tra expression in adult ISCs affects splicing of 38 transcripts by at least 5 different mechanisms. The outcome of each of the alternative splicing mechanisms is shown in yellow for a representative gene. d, Adult-restricted downregulation (RNAi lines) or misexpression (UAS lines) of tra targets in adult ISCs/EBs by means of esg-Gal4, tubGal80TS. Genes normally repressed in female progenitors in a traF-dependent manner were downregulated in males (top row) and/or misexpressed in females (bottom row). Genes upregulated in female progenitors in a traF-dependent manner were downregulated in females (bottom row). Adult-restricted downregulation of Idgf1 and Spn88Eb reduces the number of mitoses (pH3-positive cells) in DSS-treated females. Conversely, rdo misexpression inhibits DSS-induced ISC proliferation in females. Adult-restricted downregulation of other traF targets in the same conditions does not affect ISC proliferation in either males or females. e, Male controls for Fig. 3c. In contrast to their effects on females, adult ISC/EB-restricted misexpression of rdo or downregulation of Idgf1 and Spn88Eb does not reduce the percentage of midgut area covered by esg-positive cells in DSS-treated males (DNA: DAPI, in blue; ISC/EB marker: GFP, in green). n denotes the number of guts (d) that were analysed for each genotype. Results were combined from at least two independent experiments. See Supplementary Information for full genotypes.
Extended Data Figure 8 Effects of the sexual identity of adult ISCs on midgut size and reproductive plasticity.
a, The number of cells in the R3a-b and R4a midgut regions, defined by expression of Cut and MvlNP2375 respectively (as described in ref. 86), is higher in females, and can be significantly reduced in females to numbers comparable to those found in males after 20 (but not 3) days of adult-specific downregulation of tra in intestinal progenitors (achieved by esgTS-driven tra downregulation initiated after the phase of midgut post-eclosion growth, see Methods for details). No effects are apparent following downregulation in males. Representative images of these midgut regions (labelled in red with Cut or in green with MvlNP2375,esgTS-driven GFP) are shown to the right for each genotype. b, Adult ISC/EB-specific tra downregulation does not affect the number of ISCs (esg-positive, Su(H)-negative cells) in either males or females, but strongly reduces EB (esg-positive, Su(H)-positive cells) production in females. c, d, Quantifications as in a and b for midguts with adult-specific downregulation of Sxl in intestinal progenitors. c, Reduced number of cells in the R4a midgut region (top graph) and total midgut length (bottom graph) in female flies following 20 days of adult-specific and cell-autonomous masculinization of their intestinal progenitors (achieved by-down regulation of Sxl over 20 days with esg-Gal4). The same manipulation has no discernible effects in males. d, The same genetic manipulation does not affect the number of ISCs (esg-positive, Su(H)-negative cells) in either males or females, but strongly reduces EB (esg-positive, Su(H)-positive cells) production in females. e, The number of EBs (esg- and Su(H)-positive cells, bottom graph), but not ISCs (esg-positive only cells, top graph) is higher in control female flies 3 days after mating. Adult ISC/EB-specific tra downregulation abrogates the postmating increase in EBs in females without affecting EB number in males, or ISC number in males or females. f, Adult-specific Sxl downregulation in intestinal progenitors leads to a small, but significant, reduction in egg production. An unrelated manipulation that also reduces ISC proliferation by inducing differentiation of ISCs (esgTS>Notchintra, images to the right of the graph and ref. 87) also results in reduced egg production, whereas downregulation of Dsx (which does not control sex differences in progenitor proliferation) has no such effect. It should, however, be noted that esg-Gal4 is expressed in a subset of cells in the ovary niche21. Hence, the possibility that these cells contribute to the observed phenotype cannot entirely be ruled out. Images to the right show loss of intestinal progenitor cell makers esg-Gal4 and Su(H)-LacZ following expression of Notchintra in adult intestinal progenitors, indicative of loss of progenitor identity. n denotes the number of guts (a, c), ISCs/EBs (b, d, e) or female flies (f) that were analysed for each genotype. Results were combined from at least two independent experiments. See Supplementary Information for full genotypes.
Extended Data Figure 9 Effects of the sexual identity of adult ISCs on the susceptibility to genetically induced tumours.
a, The number of mitoses in Apc-ras mutant clones is larger than that of control clones in both sexes, but it is higher and dependent on Sxl in females. b, The size of Delta (Dl, the Notch ligand) null mutant (DlRevF10) MARCM clones is larger than that of control clones in both sexes, but female mutant clones are larger than male mutant clones. The graph shows quantifications of the number of cells within each clone 15 days after clone induction by heat shock, and the confocal images show representative clones (labelled in green with GFP) for each genotype. c, In tra ‘female’ mutant flies (traKO and traKO/Df(3L)st-j7) reduced Notch signalling in intestinal progenitors fails to induce the hyperplasia (quantified by the number of pH3 cells) normally observed in control females. d, Following 15 days of adult-specific downregulation of Notch (N) in intestinal progenitors, hyperplasia (quantified by the number of pH3-positive cells and also shown in representative images) is observed in female, but not male midguts. Adult-specific and cell-autonomous reversal of ISC/EB female identity—achieved by esgTS-driven downregulation of Sxl—fully prevents the hyperplasia induced by Notch downregulation in females, but has no effects on males. Confocal images show intestinal progenitor coverage of representative midgut portions for each genotype (DNA: DAPI, in blue; ISC/EB marker: GFP, in green). e, pH3 quantifications show a comparable effect for independent RNAi transgenes against Sxl and Notch. f, Adult-specific downregulation of Notch (N) signalling by ectopic expression of the downstream Notch signalling antagonist Hairless (H)38 leads to hyperplasia (quantified by the number of pH3-positive cells and also shown in representative images) in female, but not male midguts. Adult-specific and cell-autonomous reversal of ISC/EB female identity—achieved by esgTS-driven downregulation of Sxl—fully prevents the hyperplasia induced by Hairless overexpression in females, but has no effects on males. Confocal images show intestinal progenitor coverage of representative midgut portions for each genotype (DNA: DAPI, in blue; ISC/EB marker: GFP, in green). g, The number of pH3-positive cells 15 days after Notch downregulation in adult intestinal progenitors of double null mutant flies lacking dsx and fruM (dsx∆,Df(3R)Exel6179/dsx1, fruP1.LexA) is comparable to that of controls in both males and female flies. Like control flies, it is significantly higher in female flies. Virgin flies were used in all these experiments. n denotes the number of guts (a, c, d, e, f, g) or clones (b) that were analysed for each genotype. Results were combined from at least two independent experiments. See Supplementary Information for full genotypes.
Supplementary information
Supplementary Information
This file contains the full genotypes and primers used in this study. (PDF 515 kb)
Supplementary Tables
This file contains Supplementary Table 1. (XLSX 317 kb)
Rights and permissions
About this article
Cite this article
Hudry, B., Khadayate, S. & Miguel-Aliaga, I. The sexual identity of adult intestinal stem cells controls organ size and plasticity. Nature 530, 344–348 (2016). https://doi.org/10.1038/nature16953
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature16953
This article is cited by
-
Y chromosome toxicity does not contribute to sex-specific differences in longevity
Nature Ecology & Evolution (2023)
-
IFNγ-Stat1 axis drives aging-associated loss of intestinal tissue homeostasis and regeneration
Nature Communications (2023)
-
Systemic changes in cell size throughout the body of Drosophila melanogaster associated with mutations in molecular cell cycle regulators
Scientific Reports (2023)
-
The function and evolution of a genetic switch controlling sexually dimorphic eye differentiation in honeybees
Nature Communications (2023)
-
Exposure to polystyrene microplastic beads causes sex-specific toxic effects in the model insect Drosophila melanogaster
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.