Abstract
The circadian clock is a molecular timekeeper, present from cyanobacteria to mammals, that coordinates internal physiology with the external environment. The clock has a 24-h period however development proceeds with its own timing, raising the question of how these interact. Using the intestine of Drosophila melanogaster as a model for organ development, we track how and when the circadian clock emerges in specific cell types. We find that the circadian clock begins abruptly in the adult intestine and gradually synchronizes to the environment after intestinal development is complete. This delayed start occurs because individual cells at earlier stages lack the complete circadian clock gene network. As the intestine develops, the circadian clock is first consolidated in intestinal stem cells with changes in Ecdysone and Hnf4 signalling influencing the transcriptional activity of Clk/cyc to drive the expression of tim, Pdp1, and vri. In the mature intestine, stem cell lineage commitment transiently disrupts clock activity in differentiating progeny, mirroring early developmental clock-less transitions. Our data show that clock function and differentiation are incompatible and provide a paradigm for studying circadian clocks in development and stem cell lineages.
Similar content being viewed by others
Introduction
Circadian rhythms are 24-h cycles of physiological activity driven by the circadian clock, a molecular pacemaker found in nearly all cells of the body1,2. The circadian clock promotes health by coordinating tissues in the body to anticipate daily environmental changes3. In many animals, the circadian system is hierarchical, consisting of a central pacemaker in the brain and peripheral pacemakers located in other organs4. How the circadian clock develops is not well understood, but it arises during early embryogenesis in fish5 and late fetal and post-natal development in humans6. The central pacemaker in mice arises earlier than clocks in the rest of the body7,8,9 which suggests that certain tissue clocks are suppressed during development10. In Drosophila melanogaster, larval behaviors such as photo-avoidance show circadian rhythms11,12,13,14,15 and the central pacemaker can be synchronized to the environment as early as first instar larva16. These observations raise questions about when and how the circadian clock emerges in specific tissues, and the genetic and/or cellular mechanisms that underlie the birth of the timing mechanism.
The circadian clock of Drosophila is a transcription-translation cycle in which the Circadian locomotor output cycles kaput or clock (Clk) / cycle (cyc) heterodimer activates gene expression while two of its targets, period (per)17 and timeless (tim)18, repress Clk/cyc activity to restart the cycle (Fig. 1A)19. A secondary loop consisting of Par-domain protein 1 (Pdp1) and vrille (vri) stabilizes these rhythms by regulating Clk1,20,21. In Drosophila, peripheral clocks can be synchronized to the environment indirectly by the central pacemaker in the brain1 and directly through the blue-light photoreceptor cryptochrome (cry)22.
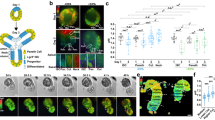
A The circadian clock comprises the transcriptional activators Clk/cyc and their repressors per/tim. The repressor vri and activator Pdp1 form a secondary feedback system and light is sensed through cry. B Drosophila development from egg through three larval instars, followed by pupation prior to eclosion as an adult. Images of the midgut from larva to adults shows dramatic growth and remodeling of the intestine, DAPI stains nuclei (black). All images shown at same scale (scale bar = 200 µm). “P” indicates posterior. C Schematic of the Clock Reporter showing binding of Clk/cyc to the minimal promoter of either tim (for the ClockTIM reporter) or per (ClockPER) located upstream of destabilized GFP (dGFP). D Images of larva, pupa, and adult show that ClockPER is expressed only in adult intestines (Myo1A>mCherry). The intestine is outlined in a dashed line (scale bar = 500 µm). Whole mount quantification of the number of flies expressing (E) ClockPER or (F) ClockTIM in the intestine throughout development. G Antibody staining showing that PER protein is only expressed in the adults and is localized to the nuclei in ClockTIM flies at ZT0 (Zeitgeber Time relative to photoperiod, ZT0=lights on, ZT12=lights off). Scale 10 µm. Histone marks nuclei. H The mRNA expression of circadian clock genes at ZT0 from 3rd instar larva to adults showing the circadian clock transcripts increase in adults. One-Way ANOVA p < 0.0001 Clk and vri, p = 0.0001 tim and Pdp1. Error bars indicate ±SEM. Each point represents one replicate (20 intestines). Representative images of two replicates. Full statistics are shown in Supplementary Information. Related to Supplementary Figs. 2–3. Source data are provided as a Source Data file. L1 1st Instar Larva.
To address the question of how circadian clock activity is established during development, we tested a Drosophila tissue with a simple lineage23,24, well-established developmental stages25,26, and adult circadian clock activity27,28. The adult intestine is derived from a population of cells known as adult midgut precursors (AMPs) that proliferate throughout larval development29,30,31,32 and pupation30,33,34, until the intestine matures three days after emergence as an adult (eclosion)29,31,35. The intestine consists of intestinal stem cells (ISCs) which divide into progenitor cells, known as enteroblasts (EBs) or enteroendocrine mother cells, that then differentiate into enterocytes (ECs) or enteroendocrine cells (EEs), respectively23,24,36. In the adult, the circadian clock controls ISC mitosis during regeneration27, and we have previously shown that epithelial cells of the intestine have clock function28.
Our results demonstrate that clock activity arises only after eclosion where it is synchronized to the environment by photoperiod and feeding over the first three days of adulthood. We characterize the transcriptome of single cells in the Drosophila intestine during adult maturation and clock formation and identify key regulators of this developmental transition. We propose a model where the ISCs are the first cells to have robust clock gene expression that is disrupted during cellular differentiation to be resumed in specialized tissue cells. We further suggest that complete clock gene networks are absent in EEs and EBs, which express only a subset of clock genes. Insects represent the most abundant and diverse species of animal on Earth, hence, our observations are significant in understanding how a critical physiological process is established in these animals and functions in their adulthood. The maturation of the Drosophila intestinal clock is also reminiscent of mammalian / human development, where tissue clocks are synchronized to daily timing postnatally. Thus, our results establish a paradigm for the birth of circadian timekeeping in animal tissues by providing a transcriptomic framework of clock development at the single cell level.
Results
The circadian clock emerges in the adult intestine
During post-embryonic development the intestine undergoes significant growth and remodeling (Fig. 1B). We first determined if the circadian clock affects intestinal development by comparing isogenic wildtype and per01 mutant flies that exhibit no clock rhythms. Intestinal size, cell density, and the number of cells in AMP / PC islets do not differ significantly in per01 mutants (Supplementary Fig. 2A, B), indicating that intestinal development is not affected in the absence of circadian clock activity. To determine when Clk/cyc transcriptional activity begins, we used two different reporter constructs (ClockPER and ClockTIM) inserted on different chromosomes that use destabilized GFP (dGFP) to visualize Clk/cyc transcriptional activity on per and tim promoters with ~1 h temporal resolution28 (Fig. 1C). The intestine was visualized using the GAL4/UAS system (Myo1A>mCherry) through the marker Myosin 31DF (Myo1A) that is expressed in both larval and adult intestinal cells37. No Clk/cyc transcriptional activity is present in larval (L1, L2, L3) or pupal flies, however, immediately after eclosion (adult day 1), Clk/cyc become active and remain so in later adulthood (Fig. 1D–F, Supplementary Fig. 2C, D) irrespective of sex or reproduction status (Supplementary Fig. 2E–G. To verify our reporter, we probed for per protein using both an antibody and a C-terminal GFP fusion reporter (per-AID-eGFP)38, and cry protein using an N-terminal fusion protein for cry (cry-GFP)39. In all cases, these clock proteins are absent in pupa but present in recently eclosed adults (Fig. 1G and Supplementary Fig. 3A–C). We also verified that the clock is not present in larva and pupa, in a circadian phase opposite to the adults, by checking expression at different timepoints (Supplementary Fig. 3D). These results indicate that circadian clock activity begins only after pupal metamorphosis in the Drosophila intestine.
We next used RT-qPCR to examine the transcriptional expression of the core clock genes (Clk, cyc, per, tim), the secondary stabilizing loop (Pdp1, vri), the blue-light photoreceptor (cry), and doubletime (dbt), a kinase that regulates per to reset Clk/cyc driven transcription40,41. The intestinal expression of Clk, tim, Pdp1E, and vri increase throughout development to peak in adulthood, whereas dbt, cyc, per, and cry expression increase earlier in development before the stage when Clk/cyc activity exists (Fig. 1H and Supplementary Fig. 3E). Therefore, we hypothesized that the changes in clock activity are likely due to changes in Clk, tim, Pdp1 and/or vri.
When we looked more closely at Clk/cyc activity in the last stages of pupation we noticed few scattered GFP cells (Supplementary Fig. 3F), raising the question of which intestinal cell type expresses Clk/cyc activity first. We measured Clk/cyc activity in pupal and adult ISC/EBs (marked by esg>mCherry) and ECs (marked by Myo1A>mCherry) and found that these early ISC/EBs show similar Clk/cyc activity in the late pupa and adults, unlike ECs that have significantly higher Clk/cyc activity in adults, suggesting that the ISC/EBs establish their circadian clock first (Supplementary Fig. 3G, H). Moreover, when the circadian clock is absent in all cells except ISC/EBs (cyc0 clock-dead mutant with esg>cyc rescue) clock activity persists in the ISC/EBs indicating that these cells can develop clocks independently of the other clocks in the body (Supplementary Fig. 3I).
Daily clock rhythms are established after three days of adulthood
Since the intestinal clock emerges after pupation, we asked whether it is rhythmic immediately, with daily maxima and minima that are characteristic of circadian rhythms, or whether it needs time to synchronize to the environment. We tested wildtype CantonS fly intestines by RT-qPCR to compare adult day 1 (immature) and day 4 (mature) gene expression patterns over a 24-h period. On day 1, the transcripts of Pdp1 and vri are not rhythmic suggesting that the transcriptional cycle is not yet present. These genes increase in rhythmicity three days later, with cry changing the timing of its initial rhythms (Fig. 2A). Of note, the expression of all clock genes, with the exception of cry, are lower on day 1 than day 4, suggesting that establishment of robust transcriptional cycles takes several days.
A Circadian clock genes are expressed in adults immediately after eclosion (day 1, shown in the schematic) but rhythms are stronger at day 4. For instance, amplitude of mRNA rhythm of Pdp1 increases, and cry timing changes, other genes similarly show differences as intestine matures. Line shows mean, day 1 is shown in a dark green dashed line, day 4 in a light green solid line. Error bars indicate ±SEM. Each point is the average of two replicates each consisting of 15–20 intestines, n = 2. Two-Way ANOVA Pdp1 p = 0.3867; vri p = 0.2637; cry p = 0.0270; tim p = 0.5207; per p = 0.2634; Clk p = 0.5399; cyc p = 0.5007. B Clk/cyc transcriptional activity in the ClockPER reporter shows daily rhythms are established robustly on day 4 with a peak around ZT0 and trough around ZT12. Cosinor fit analysis (right graph) shows arrhythmic activity on Day 1 (no cosinor curve can be fitted to the data) and 24-h rhythms on Day 4 shown in a light green solid line. Lines show mean. Error bars indicate ±SEM. Each point represents one intestine, n = 737 intestines over at least two independent replicates. C In the ClockTIM reporter rhythms show a similar trend but are noted earlier, from day 1, that phase shift to match those of ClockPER by day 4. Lines show mean, day 1 dark green dashed line, day 4 light green solid line. Error bars indicate ±SEM. Each point represents one intestine, n = 853 intestines over at least two independent replicates. Day 1 corresponds to the first light and dark cue after eclosion. D Clk/cyc activity when shifted to constant darkness prior to ZT0 on Day 4 shows free-running rhythms over the first two days in constant darkness. The vertical line separates the first and second days in constant darkness. One-Way ANOVA p < 0.0001. Lines show mean. Error bars indicate ±SEM. Each point represents one intestine, n = 198 intestines over two independent replicates. E Protein expression of per (PER-AID-eGFP) at ZT0 and ZT12 shows that there is no per protein expression in larva or pupa, but per is expressed in day 1 or day 4 adults with clear nuclear (ZT0) and cytoplasmic (ZT12) staining established by day 4. Scale 10 µm. DAPI marks nuclei. Representative images of two replicates. Full statistics are shown in Supplementary Information. Related to Supplementary Figs. 3–4. Source data are provided as a Source Data file.
The gradual emergence of transcriptional rhythmicity suggests Clk/cyc transactivation is not synchronized until the intestine matures. To test this, we imaged the ClockPER and ClockTIM reporters and measured fluorescence intensity in the whole intestine following pupation every 3-h over the first four days of adulthood (Fig. 2B, C and Supplementary Fig. 4A, B). When the adult ecloses, Clk/cyc transcriptional activity is present, but its rhythms are not robust 24-h circadian oscillations until day 4 when these consolidate to exhibit a maximum in the morning (ZT21-ZT0) and minimum in the evening (ZT9-ZT12) characteristic of the Drosophila intestine28. In each individual midgut, we did not notice major differences between cells which would indicate that the clocks in individual cells are at drastically different phases. To further test the synchrony between individual cells, we quantified EC fluorescence and noted that, although weaker rhythms are present at day 1, robust EC-specific rhythms are consolidated on day 4 (Supplementary Fig. 4C). This suggests Clk/cyc activity at both per (Fig. 2B) and tim (Fig. 2C) promoters synchronize over the first three days in the adult intestine.
A defining characteristic of circadian rhythms is their ability to continue in the absence of environmental cues such as photoperiod42. Accordingly, ClockTIM intestines were examined when flies were shifted to constant darkness (DD) prior to ZT0 on day 4 and tracked for 48-h (Fig. 2D). The rhythms are similar to those under LD conditions, with a maximum in the morning (CT21-0) and minimum in the evening (CT12), demonstrating that Clk/cyc activity is free-running at day 4.
In a functioning circadian system, clock proteins shuttle between the nucleus and cytoplasm43. We tested a GFP reporter, per-AID-eGFP38 at two timepoints ZT0 and ZT12 that represent nuclear and cytoplasmic localization of per, respectively27. Larva and pupa do not show any expression of per consistent with our qPCR and reporter analysis; the immature adult (day 1) shows signal at ZT0 that remains at ZT12, suggesting that the protein shuttling is not yet established, while the mature adult (day 4) shows strong nuclear signal at ZT0 but not at ZT12 (Fig. 2E). Taken together, our results show that circadian clock development in the intestine has three distinct phases: (1) embryogenesis to late pupa when the circadian clock circuit is absent, (2) between adult day 1 to 3 when Clk/cyc initiate clock rhythmicity but not yet fully synchronized to the environment, and (3) day 4 when the circadian clock is synchronized and rhythmic in the mature intestine.
Clock gene expression increases in ISCs and ECs during intestinal maturation
Previous studies have not been able to examine circadian clock development in individual cells of a tissue, hence it is not clear if clock components mature homogenously, with individual cells gradually expressing all clock genes, or heterogeneously, with individual cells expressing certain clock genes but not others. To further test the development of the circadian pacemaker, we profiled transcriptional changes in CantonS intestinal development over each of the three stages in clock development, from early pupa, immature adults (day 1), and mature adults (day 7–8) using single-cell RNA sequencing (scRNA-seq). Early pupal intestines do not have Clk/cyc activity, immature adult intestines have Clk/cyc activity but no rhythms, and mature adult intestines have rhythmic Clk/cyc activity; we hypothesized that clock development would increase homogenously in all intestinal cell types over these developmental stages. We recovered 5190 cells (2013 pupal, 1197 immature adult, and 1980 adult cells) that show 23 different cell states (Fig. 3A and Supplementary Fig. 5A, B), consistent with previous scRNA-seq studies of the Drosophila intestine35,44,45,46,47, capturing the changing intestinal transcriptome throughout development. Our data provides a resource for cellular development in the insect intestine.
scRNA-seq analysis of clock gene expression of the intestine of early pupa, day 1 immature adults, and day 7-8 mature adults showing (A) tSNE plots with 23 different cell populations over the integrated dataset. B Population changes are evident during three developmental stages with PupCs (green) disappearing from the pupal intestine, and ECs (dark blue) appearing in distinct clusters in the mature intestine. ISCs (aquamarine) are present at all stages, similar to EEs (light blue). For cluster assignments and markers see Supplementary Fig. 5A and Supplementary Data 4. C Multidimensional plots showing the changes in four highly expressed clock genes from early pupa to adult: tim (y-axis), cwo (x-axis), Pdp1 (color), vri (size of datapoint). Initially expression of all four genes in ISCs (first column of graphs) is low but increases during maturation. This pattern is also seen in ECs (third column). EBs and EEs do not show these increases, cells remain clustered together at low levels of expression of all four genes. D PupCs also show low clock gene expression. See Supplementary Information for correlation matrices of multidimensional plots. Related to Supplementary Fig. 5, Supplementary Data 1–4. ISC intestinal stem cell, EB enteroblast, EC enterocyte, EE enteroendocrine cell, PupC pupal cell.
During the transition from pupa to mature adult, the cell population of the Drosophila intestine undergoes cellular changes (Fig. 3B). The ISCs and EEs are present at all stages but ECs are more restricted to later stages. A population of cells we denote as Pupal Cells (PupC) decrease in the immature adult and are completely absent in the mature adult. PupCs express some genes found in the EEs and ECs of the adult (Supplementary Data 4)45 and are likely derived from AMPs but represent a separate cell type only present in pupae (Supplementary Fig. 5C), consistent with previous reports that show transient pupal midgut cells disappear during metamorphosis25. Several subtypes of differentiated ECs are not present in the pupae or immature adult and are only present in the mature adult, consistent with the maturation and growth of the Drosophila intestine48. To assess circadian gene expression in these cells, we surveyed gene counts in individual cells (Supplementary Fig. 5D and Supplementary Data 1–3). We were able to detect all core clock components (Supplementary Fig. 5D) whose expression at the population level increases as pupa transition to mature adults consistent with the RT-qPCR data (Fig. 1H and Supplementary Fig. 3E). We detected the genes per, Clk, and cyc at much lower levels than tim, Pdp1, vri, dbt, and cwo, a repressor of Clk/cyc. We therefore used the higher-expressed clock genes as a readout of clock development.
Previously, we reported that ISCs, EBs, and ECs, but not EEs have Clk/cyc transcriptional rhythms28. We therefore asked whether individual cells, including EEs, during these developmental stages express certain clock genes, but not the complete clock system. To test this, we focused on clock target genes that are more highly expressed and also feedback to regulate the core clock (cwo, tim, Pdp1 and vri). We graphed all four genes in a multi-dimensional fashion to simultaneously determine changing expression of all four in individual cells (tim is y-axis, cwo is x-axis, vri is size, Pdp1 is color). Individual ISCs show low expression of all four genes in the pupa, increasing co-expression in the immature adults, that further increases in the mature adults (Fig. 3C). This suggests that the arrhythmic Clk/cyc activity on day 1 is, at least in part, due to low expression of the circadian clock program in individual ISCs. Individual ECs show very similar patterns of change, gradually single cells develop that express all four clock genes at higher levels. In contrast, clock genes are not expressed in individual EBs and EEs in the same manner. First, these do not show the robust expression of multiple clock genes at any developmental stage including the mature intestine. For example, only 10% of mature adult EBs coexpress 3–4 of the clock genes compared to 98% of mature adult ISCs. Second, in many cells one or two clock genes are high (Supplementary Fig. 5D), but the remainder are low, suggesting clock components may have non-clock functions in specific subtypes of EEs or EBs but are not co-expressed as part of a circadian expression program. The highest clock gene expression is present in ISCs, that increase expression in the immature and mature adult intestine, whereas the other intestinal cell types showed lower expression levels. Finally, individual PupCs do not express clock genes or express few at very low levels, which is consistent with the absence of Clk/cyc activity in the pupal intestine (Fig. 3D). We conclude that ISCs and ECs increase clock gene expression after pupation, supporting a model where clock gene expression that is absent earlier in development increases in single cells homogenously to reach its maximum in the mature adult intestine.
Hormonal regulators of clock emergence
The sudden emergence of Clk/cyc activity in the immature adult intestine (Fig. 1D–F) suggests that either a regulatory factor initiates the expression of circadian clock genes in the adult, or else suppresses them in the pupa, until the appropriate developmental stage is completed. Hormone nuclear receptors control many aspects of Drosophila physiology and metamorphosis49,50 and are intimately connected with circadian clock function51. We therefore hypothesized that clock development is coordinated by hormone nuclear receptor signaling in the developing intestine. We used SCENIC52, a bioinformatic approach, that evaluates scRNA-seq transcriptomes to identify active gene networks and infer the underlying transcriptional regulators, to analyze pupal, immature, and mature adult cells. Identification of cell-specific transcription factors revealed enrichment of klu and Sox21a in ISCs, known to regulate ISC differentiation/function45,53,54,55, the Notch target E(spl) in ISC/EBs, known to drive ISC differentiation56,57, nub/Pdm1 in ECs, a well-known marker of this cell type23,24, tap in EEs58, known to promote EE cell fate59 (Fig. 4A). Hence SCENIC is able to identify cell-specific transcriptional networks in the Drosophila intestine accurately.
A SCENIC analysis of differentially expressed regulons that distinguish individual cell populations show clear changes in the transcriptional programs of different intestinal cell types (klu and Sox21a in ISCs, nub in ECs, tap in EEs). B Differential regulon expression focusing on the pupal-to-adult transition in ISCs shows that specific transcriptional pathways change during ISC development (gce/met, Pdp1, Stat92E are increased; EcR, GATA are decreased). Scaled AUC values are shown, ##g indicates the number of genes enriched in each regulon’s network. C The tSNE plot for early pupa and immature adult cells highlighting the ISC cluster used for SCENIC analysis, AUC values shown on a tSNE and in histograms showing population distributions based on regulon expression for the nuclear receptors gce and the clock gene Pdp1. Related to Supplementary Fig. 6 and Supplementary Data 4.
To infer regulators of clock differentiation we focused on the ISC population, since it is present at all three stages in intestinal development (Fig. 3B and Supplementary Fig. 5A). We evaluated differences in regulon activity between the early pupa and immature adults to specifically address the changes occurring during these developmental stages as the clock first emerges (Fig. 4B and Supplementary Data 4). This analysis identified increased activity of 13 transcription factors, and decreased activity of 17 others during pupation. These include known ISC regulators Stat92E60,61,62,63,64, and GATA65, the hormone signaling factors gce/Met (germ cell-expressed bHLH-PAS/Methoprene-tolerant)66,67, and EcR (ecdysone receptor)68,69,70 that are active during metamorphosis69,71,72, and the clock gene Pdp1. Many other factors were identified that are specific to different cell types in the developing intestine. Our dataset provides a resource for the global transcriptional changes that occur during the growth and development of the Drosophila ISC model system.
Drosophila pupation consists of sequential hormonal pulses with rhythms in adult emergence occurring through circadian control of the EcR73. We find that EcR and the nuclear receptor ftz-f1 (fushi tarazu transcription factor 1), are downregulated in the immature adult ISCs, whereas gce and Met, both of which are involved in Juvenile hormone signaling during metamorphosis66,67, are upregulated (Fig. 4B, C). Some of these differentially expressed regulons also show clear bimodal distributions indicating that there are distinct cell populations, with and without activity of these transcription factors (i.e. gce, Pdp1, Fig. 4C). This analysis provides a set of potential candidates that regulate the initiation of circadian clock activity.
To test whether these and other hormone nuclear receptors regulate clock development, we performed a screen by depleting genes in a cell-specific manner. Using our scRNA-seq analysis as a guide, we depleted components of hormone signaling in ISC/EBs (esgTS, 17 genes) using RNAi in ClockTIM reporters during pupation (Fig. 5A and Supplementary Data 5). We reasoned that the loss of positive regulators of clock differentiation would delay Clk/cyc activity, while negative regulators would hasten it. We found that (as predicted by SCENIC) Clk/cyc activity was reduced by Hr78, Hnf4, and gce knockdown, however, others such as Met, and ftz-f1 did not (Fig. 5A). Several other nuclear receptors were identified that also decrease Clk/cyc activity, but none were found that show an increase. To further test the requirement for hormonal signaling in activating clock gene expression, we overexpressed a subset of nuclear receptors identified in both the SCENIC analysis (Fig. 4B) and tested by RNAi (Fig. 5A). None of these were sufficient to accelerate Clk/cyc activity in pupal stages, suggesting that these pathways are required for circadian maturation but alone are not sufficient to prematurely drive clock emergence (Fig. 5B). However, after eclosion Clk/cyc activity is significantly higher when Hnf4 or Hr78 are overexpressed, suggesting an important role in regulating intestinal clock development (Fig. 5B). Of note, EC reporter expression is also increased when these genes are overexpressed in ISCs, possibly due to the higher clock activity in their precursors. Since common regulatory pathways might affect subsequent clock activity in differentiating ECs as well, we retested EC-specific knockdowns of the same genes tested in ISC/EBs. Consistent with ISC/EBs, components of Hnf4 signaling in ECs decreased Clk/cyc activity on the first day after eclosion, compared to the control (Fig. 5C).
A Knockdown of nuclear receptors in ISC/EBs show that loss of hormone signaling transduction decreases Clk/cyc activity. Scale 500 µm. DAPI marks nuclei. One-Way ANOVA p < 0.0001. B Overexpression in ISC/EBs of candidates shows that Hnf4 and Hr78 upregulate Clk/cyc activity compared to control midguts. Scale 500 or 10 µm for whole gut and close-up images, respectively. DAPI marks nuclei. One-Way ANOVA p < 0.0001. C Knockdown of nuclear receptors in ECs shows loss of hormone signaling transduction also affects Clk/cyc activity in ECs. Scale 500 µm. DAPI marks nuclei. We also noticed that EC knockdown of ftz-f1, and ISC/EB overexpression of Eip78C, fewer flies eclosed indicating a developmental role for these genes. In all graphs: Lines show median and quartiles. One-Way ANOVA p < 0.0001, multiple comparison, p-value < 0.05 (significant groups are shown in maroon and marked with an asterisk) compared to the control (Luc shown in blue) are shown. Scale 500 µm. DAPI marks nuclei. Representative images of two replicates. Full statistics are shown in Supplementary Information. Related to Supplementary Fig. 6 and Supplementary Data 4–5. Source data are provided as a Source Data file.
Our SCENIC analysis also suggests that Pdp1 may be a factor, however, Pdp1 loss (Pdp13135 mutant74) does not affect initiation of Clk/cyc activity (Supplementary Fig. 6A). This suggests that Pdp1 is not required for clock development, consistent with a role for Pdp1 in regulating clock outputs without being necessary for Clk expression75. Together, these data are consistent with the notion that multiple hormone-signaling pathways in the intestine during pupal development regulate the emergence of the clock and highlight Hnf4 signaling of particular importance to circadian clock development.
The EC lineage reveals heterogeneity in clock activity during differentiation
EBs are progeny of ISCs that differentiate into ECs23,24, but do not express clock genes robustly (Fig. 3C). We therefore tested clock gene expression in the mature adult ISC to EC lineage. To distinguish between the steps in differentiation, we re-clustered and ordered adult cells from ISCs (cluster 0), EBs (clusters 2, and 3), and three EC populations (clusters 7, 8, 14) to reconstruct these stages in differentiation (Supplementary Fig. 6B). We then followed the longest lineage (ISC-0 to EC-7) to determine whether clock gene expression changes during EC differentiation (Fig. 6A). The expression of a control housekeeping gene, RpL32, is present in all clusters, whereas the gene klu that correlates with the ISC identity45,76, and the EC marker gene Amy-p45 are enriched in their respective populations (Fig. 6B). Clock gene expression was mapped on the same lineages and is reduced during the different stages of EB differentiation: Pdp1, tim, vri, and cwo are initially high in ISCs, then temporarily reduced in the EB populations, re-emerging in ECs when these complete differentiation (Fig. 6B, C). Since nuclear receptors play a role in activating Clk/cyc during development, we tested the expression of hormone signaling components in ISC lineages. Hnf4, Eip75B, and gce also show high expression in ISCs, low in EBs, returning in ECs (Supplementary Fig. 6C). This suggests that nuclear receptors are correlated with clock activity during adult ISC differentiation.
A A subset of clusters from the mature adult intestine (0,2,3,7,8,14) were assembled into a lineage from earliest cells (ISC = cluster 0) to differentiated ECs in pseudotime. See Supplementary Fig. 6B for more details. B Expression of a housekeeping gene, RpL32, shows it is expressed in all cell populations, whereas ISC-specific klu and EC-specific Amy-p are restricted to their respective populations. Mapping clock genes such as Pdp1 shows their expression in these lineage changes. C Graphs show cellular expression levels in this lineage of RpL32, compared to cell-specific genes (klu and Amy-p), and clock genes (Pdp1, tim, vri, cwo). RpL32 is expressed throughout, klu only in ISCs, Amy-p only in differentiating EBs and ECs. Clock genes show initially high expression in mature ISCs which lowers in the transient differentiating EBs, to increase again in the EC. Lines indicate median and quartiles. Kruskal-Wallis test, full statistics are shown in Supplementary Information. D Flp/out clones show a mixture of basally located GFP+ and GFP- small cells, suggesting that Clk/cyc activity is heterogeneous in either ISCs and/or their progeny. Arrowhead marks GFP+ cell, arrow marks GFP- cell. Scale bar 10 µm. DAPI marks nuclei. Additional clones are shown in Supplementary Fig. 6D, E. Using mCherry expression to mark (E) ISCs, (F) EBs, and (G) ECs, specifically, Clk/cyc activity (ClockPER) is present in ISCs, absent in EBs, and strongest in ECs. Outline indicates cell of interest marked with mCherry. Scale bar 10 µm. DAPI marks nuclei. An example of each mCherry+ cell is outlined. H Schematic summarizing the observed decrease in Clk/cyc activity and clock gene expression during differentiation. Representative images of two replicates. Related to Supplementary Fig. 6 and Supplementary Data 4.
These data indicate that clock genes are repressed in the ISC-to-EC lineage, hence we predicted that individual ISC clones should show Clk/cyc active and inactive cells. We used CD2-flp/out flies, where each CD2-marked clone consists of an initial marked ISC and all of its progeny, to lineage trace individual ISCs with a ClockTIM reporter. The clones revealed large GFP+ ECs, and a mixture of small GFP+ and small GFP- cells, consistent with the idea that a small GFP + ISC produces small GFP- EBs that go on to differentiate into large polyploid GFP+ ECs (Fig. 6D and Supplementary Fig. 6D, E)28. We further tested this idea by examining Clk/cyc activity specifically in only ISCs (esgTS, Su(H)GBE-Gal80>mCherry), only EBs (Su(H)GBE>mCherry), or only ECs (Myo1ATS>mCherry). We predicted that EBs should show lower Clk/cyc activity than their founder cells or their fully differentiated progeny. Indeed, Clk/cyc activity is present in ISCs, absent in EBs, and highest in ECs (Fig. 6E–G). These data are consistent with our scRNA-seq analysis and suggest the transient disappearance of circadian clock activity during lineage commitment of ISC to ECs in the adult intestine (Fig. 6H).
Photoperiod and feeding synchronize the maturing intestinal circadian clock
Our results indicate that the intestinal circadian clock gene expression is established during development, however, it is not clear what factors cause the intestine to exhibit synchronous circadian rhythms. The intestine completes its growth and development after adult emergence from pupation35,48, while it is being synchronized to the environment (Fig. 2B, C). We have previously shown that the Drosophila intestinal clock is synchronized by photoperiod light cycles, with food ingestion also playing a modulating role in timing daily rhythms28. We first asked if photoperiod would be sufficient to synchronize the immature intestinal clock. Flies were completely starved for the first three days of adulthood following pupation and provided food only at ZT0 on adult day 4 when clock synchronization is complete. Even though intestinal size was reduced by lack of nutrients (Supplementary Fig. 7A, B), Clk/cyc activity was robust, and synchronized rhythms emerged one day earlier (day 3 instead of day 4, compare Fig. 7A to Fig. 2C), suggesting that feeding and/or intestine growth delays clock entrainment.
A ClockTIM flies that receive only water for the first three days after pupation show robust rhythmic Clk/cyc activity earlier: by day 3 rather than day 4, suggesting the absence of food more quickly synchronizes a rhythmic clock. Line shows mean. Error bars indicate ±SEM. Each point represents one intestine, n = 595 intestines over two independent replicates. One-Way ANOVA p < 0.0001. B ClockTIM;cry01 mutant flies that cannot sense photoperiod light display arrhythmic Clk/cyc transcriptional activity until day 4 when these are established, similar to controls. This suggests food in the absence of photoperiod can also synchronize a rhythmic clock. Line shows mean. Error bars indicate ±SEM. Each point represents one intestine, n = 570 intestines over two independent replicates. One-Way ANOVA p < 0.0001. C Alternating days of feeding and starving (water only) shows arrhythmic Clk/cyc activity whereas restricted feeding for 6 h a day in ClockTIM;cry01 mutants synchronizes intestinal Clk/cyc rhythms that peak ~6 h after feeding. Ad libitum measurements (control) from Fig. 7B are replotted in gray to show difference. Line shows mean. Error bars indicate ±SEM. Two-Way ANOVA Feed/Starve p = 0.0047; RF5-11 and RF0-6 p < 0.0001. Fasting/Starvation times outlined in light blue rectangle(s). Full statistics are shown in Supplementary Information. Related to Supplementary Fig. 7. Source data are provided as a Source Data file.
We then performed the opposite experiment, in which photoperiod is absent and feeding is present, by using cry01 mutants that are unable to detect light to synchronize clock function77. We predicted that feeding would synchronize circadian rhythmicity. Without photoperiod, cry01 intestines exhibit arrhythmic Clk/cyc activity during the first three days but show Clk/cyc activity at day 4 onward similar to wildtype controls (Fig. 7B compare to Fig. 2C). Since light-responsive feeding behavior occurs in cry01 flies78 these data suggest that feeding alone can also complete circadian clock synchronization in the intestine. To further test this, we restricted feeding in cry01 flies post-pupation to the morning only (ZT0-6), evening only (ZT5-11), or subjected them to alternate days of feeding and starvation to disrupt feeding rhythmicity. We predicted that the timing of food consumption would alter the maxima and minima of intestinal circadian rhythms. Indeed, under these conditions the rhythms of cry01 intestines were shifted to peak around 6 h after feeding, or rendered non-circadian when disrupted (no clear 24-h period) (Fig. 7C). In contrast, control flies that have the ability to detect photoperiod do not show a shift in Clk/cyc activity on restricted feeding, suggesting that photoperiod is dominant over feeding if both environmental cues are detected (Supplementary Fig. 7C). We further tested if periods of fasting would affect clock rhythms once these are established in adults and noted that these do perturb the clock if photoperiod is detected (Supplementary Fig. 7D, E). We conclude that the combination of daily photoperiod and feeding cycles together synchronize the timing of intestinal circadian transcription cycles during clock maturation.
Discussion
There are many questions about how circadian rhythms develop in the body10,79 and, in particular, connections between circadian rhythms and stem cells are poorly understood80,81. We show that as the intestine grows and differentiates clock gene transcription is negatively regulated (Figs. 1, 3); ISCs differentiating into EB precursors also do not express clock genes (Fig. 6). Our study sheds light on two processes: 1) the emergence of circadian clock function in the stem cells of the intestine and 2) suppression in their immediate progeny as these establish a differentiated cell state. These data suggest that the clock and differentiation are incompatible in the intestine and are reminiscent of a recent report showing clock function is incompatible with mouse somite development82. Our study characterizes the development of the circadian clock in the intestine but this framework may be extended to other organs of the body.
Circadian clock development has been tested in several animal species. In zebrafish, clocks emerge during the first stages of embryogenesis5, similarly, in Drosophila clock emergence was shown to occur early in development16. However, these initial studies of Drosophila clock development focused on circadian rhythms in the brain rather than other tissues in the body12,16. It was recently found that Drosophila larval tissues do not express the clock gene cry until adulthood39, and circadian control of the prothoracic gland also occurs in pupation83, consistent with our results (Fig. 1). Of note, we find that Clk/cyc activity is initiated in the spiracles and prothoracic gland during larval development, and the heart during pupation (unpublished observations KP, PK). This suggests that circadian timing in the Drosophila central clock emerges earlier and timing in other tissues emerges later. In chicks, even though clock expression occurs early in development, it is patchy during early stages before becoming more widely expressed84. In mice and rats, where circadian development is best studied, early embryonic cells do not have clock rhythms85,86. The central pacemaker in the brain emerges during late embryogenesis9,87,88, and other tissues such as the heart89, kidney90, and colon7 emerge even later. These mammalian tissue clocks are present just before birth and their synchronous rhythms mature at different rates postnatally91, particularly in the colon that (like our study) shows the intestine has a relatively slow clock to develop in the body7. Overall, data from a wide variety of species are consistent with our observations. Circadian clock function is also tissue-specific, and thus its emergence may be tissue-specific, eventually coalescing into complete circadian rhythmicity throughout the body at the appropriate stage of development. The larval stages where insects eat almost continuously may be incompatible with a daily timing system in the digestive tract that dictates activity/rest cycles.
Our study is the first to examine the emergence of animal tissue clocks at the single cell level, revealing that stem cells are the first to pass clock status to their differentiating progeny. The transient loss of clock gene expression as cells transition between discrete fates has been previously overlooked when using population-based methods such as RNA-seq or RT-qPCR. Thus, our study provides a paradigm of how clocks may develop in non-neural tissue cells after the central clock is established. We have used the expression of several clock components simultaneously to estimate the developmental timing of clock rhythms. It is important to recognize that the expression of clock genes alone does not confirm such rhythms are present. Indeed, we find that clock gene expression is present in the early adult intestine but is not robustly synchronized and rhythmic until several days later (Figs. 1–2). The early clock in the zebrafish circadian system also takes 3–4 days to be established, with the secondary feedback system and output genes rhythmic later in development92,93. This 2–4 day delay between gene expression and daily cycling also appears in the mouse kidney90 and central clock in the brain9.
Elegant studies in mouse embryonic cells have shown that translational regulation of Clock (the mouse ortholog of Clk) is a rate-limiting step in circadian development89. Our results are in agreement with this (Fig. 1H), however, in Drosophila we identify the component Pdp1 as another potential factor (Figs. 1H and 4C). This is consistent with a Clk-based transcriptional model because Pdp1 positively regulates Clk expression, hence Clk expression and transcriptional activity may be a conserved circadian component that is required for rhythmicity. For instance, since Pdp1 and vri maintain rhythmic Clk expression20, we note that their developmental maturation over days 1–4 may explain the initially low amplitude rhythms in Clk (Fig. 2A). Although we find that the loss of Pdp1 does not affect the timing of intestinal clock emergence, in Drosophila, it has been shown that ectopic expression of Clk can prematurely induce clocks outside the pacemaker94,95. Future work to characterize this potential mechanism in ISCs will be informative, as it may be a switch that initiates daily tissue timing in developing tissues at different times. Previous studies have shown that in the mouse liver, Hnf4 is essential for Clock/Bmal1 rhythmic activity96, and our results suggest that it also regulates the development of the Drosophila intestinal clock (Fig. 5). In addition, environmental factors such as the microbiome might play a role in maturing both the tissue and its circadian rhythms, although we note that it was recently reported that in the adult midgut the microbiome does not appear to show rhythmicity97.
This dataset provides single-cell characterization of insect intestinal metamorphosis and circadian clock development. Because insects utilize circadian rhythms to coordinate their pupation timing98, these results are informative in understanding the fundamental biology of these animals. Our analysis with SCENIC52 identified key transcription factors involved in the development of this organ, showing the existence of transitory Pupal Cells (PupCs) that express markers of early differentiation such as Sox1453. Using scRNA-seq, we were able to recover the main cell types in the intestine at all three stages and found markers to identify ISCs, EBs, EEs, ECs, and visceral muscle (VM) that are consistent with previous Drosophila intestine sequencing studies35,44,45,46,47 (Supplementary Data 4). We were not able to identify a population of enteroendocrine mother cells although the earliest ISC population includes cells enriched for piezo, a marker of the EE lineage36, therefore it is likely that these cells are located in the ISC cluster (Cluster 0, Fig. 3A). We have annotated a population of pupal cells, PupCs, that are unique to the pupal intestine and display similarities to differentiated cells including expression of MtnB and MtnD characteristic of the central intestine, Supplementary Data 4) suggesting that these cells contribute to intestine development consistent with the transient pupal midgut described previously25.
By testing clock development at the single cell level, we found that the circadian clock program is downregulated during the dynamic cellular changes that occur during differentiation. These results are counterintuitive given that the clock is thought to emerge during embryogenesis, and has in fact been demonstrated to arise in differentiating embryonic stem cells9,88, and organoids99 in vitro. Indeed, several studies have shown circadian clocks regulate cellular differentiation itself100,101,102. We therefore propose that during the transient states of cellular fate specification, clock gene expression is disrupted. A parsimonious explanation for this is that the transcriptional and epigenetic reorganization that occurs during differentiation103,104,105 simply interferes with rhythmic circadian transcription. Interestingly, a recent study observed a similar pattern of circadian disruption in thymocyte differentiation where a Per1-Venus reporter exhibits reduction in cellular stages between thymocyte precursor and fully differentiated cell106. In line with our data, this suggests that clock function requires a certain level of epigenetic stability that is not compatible with the rapid cellular fate changes that occur during development or during stem cell-driven regeneration. The Drosophila midgut provides an opportunity for future investigation of these interactions and highlight a state where cells in the body are resistant to circadian rhythms. Because both ISC differentiation (this study) and somite differentiation82 involve cell fate commitment through Notch signaling, it will be of interest to see if these principles extend to other developmental pathways and/or confirm the precise mechanisms linking the clock and the Notch pathway in the future.
In human beings, circadian rhythmicity arises postnatally: most infants take several months to establish coherent daily timing6. This is likely to reflect the complexity of establishing robust inter-cellular and inter-organ synchrony that involve coordinating environmental signals with tissue-specific clocks. Our study reveals how circadian rhythms are established in complex developing tissues and provides a system in which to investigate the birth of the circadian tissue pacemaker.
Methods
Fly Strains
Drosophila melanogaster strains in this study include: ClockPER, ClockTIM described in28. From Jadwiga Giebultowicz: CantonS and per01(isogenic with CantonS). From Chrysoula Pitsouli esg-Gal4 (II), tubGal80TS(III). From Yong Zhang PER-AID-GFP. From Steve Jean Su(H)GBE-Gal80, tubGal80ts(III). From Amita Sehgal Pdp13135(III). From Bloomington: #38424 UAS-mCherry (III), UAS-mCherry(II), myo1A-Gal4 (II), #42563 Valium20-cyc(II), cry01, #76317 CRY-GFP, #4412 act > y + >Gal4,UAS-CD2, RNAi lines: #50712 and #58286 EcR (#1 and #2), #43231 Eip75B, #26718 Eip78C, #61937 and #50868 ERR, #27659 ftz-f1, #61852 gce, #29375 and #64988 Hnf4, #51442 and #27254 Hr3 (#1 and #2), #29376 and #29377 Hr38, #33624 and #27086 Hr39, #54803 and #31868 Hr4, #31990 Hr78, #31603 Luciferase, #26205 met, #36095 tai, #36729 and #27258 Usp (#1 and #2). From FlyORF: F001915 gce, F000656 Eip78C, F000144 and F003392 Hnf4 (#1 and #2), F000423 and F003303 Hr78 (#1 and #2), F004847 and F002207 Eip75B (#1 and #2), F003474 met. Flies were balanced with yw;IF/CyO;MKRS/TM6B. Experimental genotypes are provided in Supplementary Table 1. Flies were raised on cornmeal-glucose media (1.2% w/v yeast, 0.7% w/v soyflour, 5% w/v cornmeal, 0.4% w/v malt, 0.4% v/v agar, 5.3% v/v glucose with propionic acid and tegosept) at 25 °C with 12:12 light/dark photoperiod (lights-on at ZT0). For free-running experiments flies were shifted to constant darkness (DD) during the dark-phase of the final day of LD, CT0 (Circadian Time 0) is the time when lights are expected to turn on. For larval to adult analysis, flies were only allowed to lay eggs for 5 h in the morning (ZT0-5). For adult experiments, only flies that eclosed overnight (ZT12-0) were included, no difference was noted between ZT11-12, ZT23-0, and ZT0-1 eclosion groups. Heatshocks for Flp/out clones were done with adult flies 5–14 days old for 5–10 min at 37 °C. For RNAi and overexpression screening (Fig. 5), flies were raised at 18 °C until pupal stages then vials were shifted to 29 °C and both males and females were collected. Age ranges from first larval instar to adults (day 1–14).
Food restriction
Pupa were transferred to vials containing a piece of water-moistened filter paper using a paintbrush. Flies eclosed onto this filter paper and were kept on water after eclosion either (1) until adult day 4 at ZT0 when flies were transferred to vials containing normal food or (2) for 6 h (from either ZT5-11 or ZT0-5) each day after eclosion. The alternate days of feeding and starvation (Fig. 7C) were done by allowing flies to eclose onto water-moistened filter paper, similar to the restricted feeding, then at ZT0 were flipped onto food (“F”), then the next morning at ZT0 back onto filter paper with water for starvation (“S”), then repeated F (day 3), S (day 4). This alternating cycle was necessary, since unlike the controls the cry01 flies did not survive the starvation for 2–3 days.
Developmental staging
Larva were roughly staged using the following times: 1 day AED (after egg deposition) for L1, 2 days AED is L2, 3–5 days AED is L3 (the day AED may be noted e.g., L3 day 3 refers to three days AED). Early stage pupa were analyzed at the red-eye stage (day 8 AED), and late stage pupae were analyzed at the gray to black wing stage (day 9 or 10 AED)107. These stages were verified visually to account for individual variation in developmental timings. The first day after eclosion is referred to as adult day 1 (day 9 or 10 AED).
Dissection
Flies were euthanized in 70% EtOH and then intestines were dissected using fine forceps in 1xPBS. For RNA extractions and single cell analysis, the hindgut (anterior to the Malpighian tubules) and the foregut were removed during dissection to ensure only the intestine was sampled. Dissections were completed in <1 h. Analysis from larva to adult included both males and females but unless otherwise noted, only female flies were analyzed.
Tissue preparation/antibody staining
Intestines were fixed in 4% paraformaldehyde for 40 min, washed twice in 1xPBS and stained with DAPI (1:5000 in 0.2% Triton-X100 (in 1xPBS)) for 5 min. The intestines were washed twice in 0.2% PBS-T and then mounted on slides using AlexaProLong Gold Antifade Reagent (Invitrogen). For antibody staining, intestines were fixed for 2 h in 4% paraformaldehyde and then washed twice in 1xPBS and blocked for 20 min in 1% bovine serum albumin in 0.2% PBS-T (BSA). The intestines were then incubated in primary antibody (PER (1:1500), histone (1:2000), GFP (1:2000), rat-CD2 (1:2000)) in BSA for 2 h, washed twice in 0.2% PBS-T, incubated in secondary antibody (1:2000) with DAPI (1:5000) in BSA for 1 h and then washed twice in 0.2% PBS-T (at 4 °C) and mounted on slides. Cry-GFP was stained 1-h prior to lights on (ZT23) to avoid light-induced degradation of cry39.
RT-qPCR
Approximately 15 female flies of CantonS were dissected in 1XPBS before being transferred to tubes containing RNAlater (Qiagen) on ice. RNA was extracted using the RNAMini Kit (Qiagen) with RLT buffer (Qiagen) using a Bullet Blender (Next Advance) for homogenization. cDNA was made using ISCRIPT RT Supermix (Bio-Rad) and RT-qPCR was performed using SYBR green (Bio-Rad) using the Viia7 PCR plate reader, primers: per-F TCATCCAGAACGGTTGCTACG, per-R CCTGAAAGACGCGATGGTGT27, tim-F CCAGCATTCATTCCAAGCAG, tim-R GCGTGGCAAACTGTGTTATG27, Gapdh-F CCAATGTCTCCGTTGTGGA, Gadph-R TCGGTGTAGCCCAGGATT, Clk-F CAAGTTTGGCCTCTGGCTCTC, Clk-R TACAACTAGCTCTGGGCTTCCG, Pdp1-F GAACCCAAGTGTAAAGACAATGCG, Pdp1-R CTGGAAATACTGCGACAATGTGG108, vri-F TGTTTTTTGCCGCTTCGGTCA, vri-R TTACGACACCAAACGATCGA108, cyc-F GCGCTGATGGAGTCTCACAAG, cyc-R GTAGCTGTTGTCCTTGCACCG, cry-F CACCGCTGACCTACCAAA, cry-R GGTGGAAGCCCAATAATTTGC109, dco-F TTGGGAGGAGGGTTAGCAG, dco-R TTACAATGTGGGTGCCTTGC.
scRNA-seq
Flies from CantonS were euthanized in 70% ethanol in DEPC-treated water at ZT0. Every 10 min during the 1 h dissection time, the intestines were transferred from the 1XPBS well to ice-cold 1% BSA. Once dissections were complete, the intestines were transferred to a drop of 1XPBS on the back of a dissection plate and cut into small pieces using microscissors. The fragments were then transferred to a 1.5 mL tube to a total volume of 350ul (200uL for pupa). Elastase was added to a concentration of 1 mg/mL and incubated at 37 °C for 40 min pipetting up and down 30–40 times every 10 min to dissociate the cells, then 10% BSA was added to a final concentration of 1% BSA. The samples were centrifuged at 300 x g for 15 min at 4 °C, then the pellet was resuspended in 200ul of 0.04% BSA and filtered with a 70um filter centrifuging at 300 x g at 4 °C for 1 min. Orbitrap Density Gradient was used to sort the cells and then the interface was washed with 0.04% BSA. The cell density was determined with a haemocytometer and diluted with 0.04% BSA to a density of 800cells/uL. All samples had a viability >95%. The entire preparation was completed within 2 h. The fresh samples were submitted to the High Content Analysis Core at the University of Alberta by ZT3 for 10X Genomics 3’ Library preparation and to Novogene for sequencing (NovaSeq) and then aligned to the Drosophila reference genome 6.25 using Cell Ranger (10X Genomics, version 3.0.2).
Imaging and data analysis
Slides were imaged with either a slide scanner (Zeiss, Axio Scan Z.1) or confocal microscope (Olympus, IX81 FV1000 with 60x Water Immersion Lens or Zeiss Confocal with 20x lens). Whole mount imaging and quantification were done using a fluorescence microscope (Zeiss, VertA.1) or fluorescence stereoscope (Leica M205). Images were analyzed with Zen Blue Software (Zeiss, version 2.3) and processed using Photoshop (Adobe) with adjustments consistent within a panel. Fluorescence intensity measurements are ratios of GFP:DAPI taken for the whole midgut, unless otherwise specified, as previously described110. RT-qPCR data is normalized to Gapdh1. Prism (GraphPad) was used for graphing and statistical analysis. Cosinor analysis was performed using circacompare111 and graphed using Prism (GraphPad). Schematics were prepared using Photoshop (Adobe) and Illustrator (Adobe).
Single sequencing data was analysed in R using Seurat (version 4.3.0.1)112 and pseudotime analysis was performed using Slingshot (version 2.8.0)113. The original dataset of 6835 adult, 2625 pharate, 3816 pupal cells was filtered (genes must be detected in > = 3 cells, %mitochondrial <25, 200<nFeatures<4000, 1000<nCount<100000) and the remaining 1980 adult, 1197 pharate, 2013 pupal cells were used for further analysis. The datasets were integrated (reduction = rpca) and clustered (PCs = 14, resolution = 0.5) and then annotated using differentially expressed genes (method = roc). For lineage tracing 1111 adult cells consisting of clusters 0,2,3,7,8,14 were re-clustered (PCs = 9, resolution = 0.3) or 1933 pupal cells from clusters (0–6,8–14,16,17,18,21) were re-clustered (PCs = 16, resolution = 0.3) prior to lineage tracing to further subdivide these cells. Differential gene analysis was performed using roc. SCENIC analysis (version 1.3.1)52 was performed with version 10 Drosophila motif set with minimum number of genes in each regulon = 10, then handed back to Seurat for differential gene expression analysis. In some cases, Prism (GraphPad, version 9) was used for graphing and statistical analysis. Images were processed using Photoshop (Adobe, version 23.4.2) and schematics were made using Photoshop and Illustrator (Adobe, version CS5).
Statistical tests were performed using GraphPad Prism and can be found in Supplementary Information.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The scRNA-seq data generated in this study have been deposited in the NCBI GEO database under accession code GSE230572 [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=gse230572]. The alignments were made using Drosophila reference genome 6.25 and are publicly available as of the date of publication. Source data are provided with this paper.
References
Hardin P. E. Advances in genetics. (Elsevier, 2011).
Cox, K. H. & Takahashi, J. S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 63, R93–r102 (2019).
Panda, S. Circadian physiology of metabolism. Science 354, 1008–1015 (2016).
Reppert, S. M. & Weaver, D. R. Coordination of circadian timing in mammals. Nature 418, 935 (2002).
Dekens, M. P. & Whitmore, D. Autonomous onset of the circadian clock in the zebrafish embryo. Embo J. 27, 2757–2765 (2008).
Rivkees, S. A. Developing circadian rhythmicity in infants. Pediatrics 112, 373–381 (2003).
Polidarová, L. et al. Development and entrainment of the colonic circadian clock during ontogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G346–G356 (2014).
Inada, Y. et al. Cell and tissue-autonomous development of the circadian clock in mouse embryos. FEBS Lett. 588, 459–465 (2014).
Carmona-Alcocer, V. et al. Ontogeny of circadian rhythms and synchrony in the suprachiasmatic nucleus. J. Neurosci. 38, 1326 (2018).
Umemura, Y. & Yagita, K. Development of the circadian core machinery in mammals. J. Mol. Biol. 432, 3611–3617 (2020).
Mazzoni, E. O., Desplan, C. & Blau, J. Circadian pacemaker neurons transmit and modulate visual information to control a rapid behavioral response. Neuron 45, 293–300 (2005).
Keene, A. C. et al. Distinct visual pathways mediate Drosophila larval light avoidance and circadian clock entrainment. J. Neurosci. 31, 6527–6534 (2011).
Baik, L. S. et al. CRYPTOCHROME mediates behavioral executive choice in response to UV light. Proc. Natl Acad. Sci. USA 114, 776–781 (2017).
Baik, L. S., Recinos, Y., Chevez, J. A. & Holmes, T. C. Circadian modulation of light-evoked avoidance/attraction behavior in Drosophila. PLoS ONE 13, e0201927 (2018).
Asirim, E. Z., Humberg, T.-H., Maier, G. L. & Sprecher, S. G. Circadian and genetic modulation of visually-guided navigation in Drosophila Larvae. Sci. Rep. 10, 2752 (2020).
Sehgal, A., Price, J. & Young, M. W. Ontogeny of a biological clock in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 89, 1423–1427 (1992).
Hao, H., Allen, D. L. & Hardin, P. E. A circadian enhancer mediates PER-dependent mRNA cycling in Drosophila melanogaster. Mol. Cell. Biol. 17, 3687–3693 (1997).
McDonald, M. J., Rosbash, M. & Emery, P. Wild-type circadian rhythmicity is dependent on closely spaced E boxes in the drosophila timeless promoter. Mol. Cell. Biol. 21, 1207–1217 (2001).
Darlington, T. K. et al. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science 280, 1599–1603 (1998).
Cyran, S. A. et al. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell 112, 329–341 (2003).
Glossop, N. R. et al. VRILLE feeds back to control circadian transcription of Clock in the Drosophila circadian oscillator. Neuron 37, 249–261 (2003).
Emery, P., Stanewsky, R., Hall, J. C. & Rosbash, M. Drosophila cryptochromes: a unique circadian-rhythm photoreceptor. Nature 404, 456 (2000).
Ohlstein, B. & Spradling, A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470 (2006).
Micchelli, C. A. & Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475 (2006).
Takashima, S., Younossi-Hartenstein, A., Ortiz, P. A. & Hartenstein, V. A novel tissue in an established model system: the Drosophila pupal midgut. Dev. Genes Evol. 221, 69–81 (2011).
Tepass, U. & Hartenstein, V. Epithelium formation in the Drosophila midgut depends on the interaction of endoderm and mesoderm. Development 120, 579–590 (1994).
Karpowicz, P., Zhang, Y., Hogenesch, J. B., Emery, P. & Perrimon, N. The circadian clock gates the intestinal stem cell regenerative state. Cell Rep. 3, 996–1004 (2013).
Parasram, K. et al. Intestinal stem cells exhibit conditional circadian clock function. Stem Cell Rep. 11, 1287–1301 (2018).
Jiang, H. & Edgar, B. A. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 136, 483–493 (2009).
Mathur, D., Bost, A., Driver, I. & Ohlstein, B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science 327, 210–213 (2010).
Hartenstein, V. & Jan, Y. N. Studying Drosophila embryogenesis with P-lacZ enhancer trap lines. Roux’s Arch. Dev.Biol. 201, 194–220 (1992).
Hartenstein, A. Y., Rugendorff, A., Tepass, U. & Hartenstein, V. The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development 116, 1203–1220 (1992).
Micchelli, C. A., Sudmeier, L., Perrimon, N., Tang, S. & Beehler-Evans, R. Identification of adult midgut precursors in Drosophila. Gene Expr. Patterns 11, 12–21 (2011).
Takashima, S. et al. Development of the Drosophila entero-endocrine lineage and its specification by the Notch signaling pathway. Dev. Biol. 353, 161–172 (2011).
Buchon, N. et al. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 3, 1725–1738 (2013).
He, L., Si, G., Huang, J., Samuel, A. D. & Perrimon, N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature 555, 103–106 (2018).
Morgan, N. S., Heintzelman, M. B. & Mooseker, M. S. Characterization of myosin-IA and myosin-IB, two unconventional myosins associated with theDrosophilabrush border cytoskeleton. Dev. Biol. 172, 51–71 (1995).
Chen, W., Werdann, M. & Zhang, Y. The auxin-inducible degradation system enables conditional PERIOD protein depletion in the nervous system of Drosophila melanogaster. FEBS J. 285, 4378–4393 (2018).
Agrawal, P. et al. Drosophila CRY entrains clocks in body tissues to light and maintains passive membrane properties in a non-clock body tissue independent of light. Curr. Biol. 27, 2431–2441.e2433 (2017).
Price, J. L. et al. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 94, 83–95 (1998).
Kloss, B. et al. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iε. Cell 94, 97–107 (1998).
Bruce, V. G. & Pittendrigh, C. S. Endogenous rhythms in insects and microorganisms. Am. Nat. 91, 179–195 (1957).
Saez, L., Meyer, P. & Young, M. W. A PER/TIM/DBT interval timer for Drosophila’s circadian clock. Cold Spring Harb. Symp. Quant. Biol. 72, 69–74 (2007).
Dutta, D. et al. Regional cell-specific transcriptome mapping reveals regulatory complexity in the adult Drosophila midgut. Cell Rep. 12, 346–358 (2015).
Hung, R.-J. et al. A cell atlas of the adult Drosophila midgut. Proc. Natl Acad. Sci. USA 117, 1514–1523 (2020).
Li, H. et al. Fly Cell Atlas: a single-nucleus transcriptomic atlas of the adult fruit fly. Science 375, eabk2432 (2022).
Marianes, A. & Spradling, A. C. Physiological and stem cell compartmentalization within the Drosophila midgut. elife 2, e00886 (2013).
O’Brien, L. E., Soliman, S. S., Li, X. & Bilder, D. Altered modes of stem cell division drive adaptive intestinal growth. Cell 147, 603–614 (2011).
King-Jones, K. & Thummel, C. S. Nuclear receptors-a perspective from Drosophila. Nat. Rev. Genet 6, 311–323 (2005).
Tennessen, J. M. & Thummel, C. S. Coordinating growth and maturation - insights from Drosophila. Curr. Biol. 21, R750–R757 (2011).
Zhao, X. et al. Nuclear receptors rock around the clock. EMBO Rep. 15, 518–528 (2014).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Meng, F. W. & Biteau, B. A sox transcription factor is a critical regulator of adult stem cell proliferation in the Drosophila intestine. Cell Rep. 13, 906–914 (2015).
Zhai, Z. et al. Accumulation of differentiating intestinal stem cell progenies drives tumorigenesis. Nat. Commun. 6, 10219 (2015).
Chen J., Xu N., Huang H., Cai T., Xi R. A feedback amplification loop between stem cells and their progeny promotes tissue regeneration and tumorigenesis. Elife 5, e14330 (2016).
Ohlstein, B. & Spradling, A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315, 988–992 (2007).
Guo, Z. & Ohlstein, B. Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science 350, aab0988 (2015).
Guo, X. et al. The cellular diversity and transcription factor code of Drosophila enteroendocrine cells. Cell Rep. 29, 4172–4185.e4175 (2019).
Li, H. J. et al. Intestinal Neurod1 expression impairs paneth cell differentiation and promotes enteroendocrine lineage specification. Sci. Rep. 9, 19489 (2019).
Jiang, H. & Edgar, B. A. Intestinal stem cells in the adult Drosophila midgut. Exp. Cell Res. 317, 2780–2788 (2011).
Jiang, H. et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355 (2009).
Buchon, N., Broderick, N. A., Chakrabarti, S. & Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Gene Dev. 23, 2333–2344 (2009).
Beebe, K., Lee, W.-C. & Micchelli, C. A. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev. Biol. 338, 28–37 (2010).
Lin, G., Xu, N. & Xi, R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. J. Mol. Cell Biol. 2, 37–49 (2010).
Okumura, T. et al. GATAe regulates intestinal stem cell maintenance and differentiation in Drosophila adult midgut. Dev. Biol. 410, 24–35 (2016).
Jindra, M., Uhlirova, M., Charles, J. P., Smykal, V. & Hill, R. J. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet 11, e1005394 (2015).
Abdou, M. A. et al. Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem. Mol. Biol. 41, 938–945 (2011).
Koelle, M. R. et al. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell 67, 59–77 (1991).
Li, T.-R. & White, K. P. Tissue-specific gene expression and ecdysone-regulated genomic networks in Drosophila. Dev. Cell 5, 59–72 (2003).
Truman, J. W., Talbot, W. S., Fahrbach, S. E. & Hogness, D. S. Ecdysone receptor expression in the CNS correlates with stage-specific responses to ecdysteroids during Drosophila and Manduca development. Development 120, 219–234 (1994).
Riddiford, L. M. & Truman, J. W. Hormone receptors and the regulation of insect metamorphosis. Am. Zool. 33, 340–347 (1993).
Lee, C.-Y., Cooksey, B. A. & Baehrecke, E. H. Steroid regulation of midgut cell death during Drosophila development. Dev. Biol. 250, 101–111 (2002).
Mark, B., Bustos-González, L., Cascallares, G., Conejera, F. & Ewer, J. The circadian clock gates Drosophila adult emergence by controlling the timecourse of metamorphosis. Proc. Natl Acad. Sci. USA 118, e2023249118 (2021).
Zheng, X. et al. An isoform-specific mutant reveals a role of PDP1ε in the circadian oscillator. J. Neurosci. 29, 10920–10927 (2009).
Benito, J., Zheng, H. & Hardin, P. E. PDP1epsilon functions downstream of the circadian oscillator to mediate behavioral rhythms. J. Neurosci. 27, 2539–2547 (2007).
Korzelius, J. et al. The WT1-like transcription factor Klumpfuss maintains lineage commitment of enterocyte progenitors in the Drosophila intestine. Nat. Commun. 10, 4123 (2019).
Dolezelova, E., Dolezel, D. & Hall, J. C. Rhythm defects caused by newly engineered null mutations in Drosophila’s cryptochrome gene. Genetics 177, 329–345 (2007).
Seay, D. J. & Thummel, C. S. The circadian clock, light, and cryptochrome regulate feeding and metabolism in Drosophila. J. Biol. Rhythms 26, 497–506 (2011).
Vallone, D., Lahiri, K., Dickmeis, T. & Foulkes, N. S. Start the clock! Circadian rhythms and development. Dev. Dyn. 236, 142–155 (2007).
Brown, S. A. Circadian clock-mediated control of stem cell division and differentiation: beyond night and day. Development 141, 3105–3111 (2014).
Benitah, S. A. & Welz, P.-S. Circadian regulation of adult stem cell homeostasis and aging. Cell Stem Cell 26, 817–831 (2020).
Umemura, Y. et al. Circadian key component CLOCK/BMAL1 interferes with segmentation clock in mouse embryonic organoids. Proc Natl Acad Sci USA 119, e2114083119 (2022).
Di Cara, F. & King-Jones, K. The circadian clock is a key driver of steroid hormone production in Drosophila. Curr. Biol. 26, 2469–2477 (2016).
Gonçalves, L. et al. Circadian clock genes Bmal1 and Clock during early chick development. Dev. Dyn. 241, 1365–1373 (2012).
Yagita, K. et al. Development of the circadian oscillator during differentiation of mouse embryonic stem cells in vitro. Proc. Natl Acad. Sci. USA 107, 3846–3851 (2010).
Kowalska, E., Moriggi, E., Bauer, C., Dibner, C. & Brown, S. A. The circadian clock starts ticking at a developmentally early stage. J. Biol. Rhythms 25, 442–449 (2010).
Sládek, M. et al. Insight into molecular core clock mechanism of embryonic and early postnatal rat suprachiasmatic nucleus. Proc. Natl Acad. Sci. USA 101, 6231–6236 (2004).
Landgraf, D., Koch, C. E. & Oster, H. Embryonic development of circadian clocks in the mammalian suprachiasmatic nuclei. Front. Neuroanat. 8, 143 (2014).
Umemura, Y. et al. Involvement of posttranscriptional regulation of Clock in the emergence of circadian clock oscillation during mouse development. Proc. Natl Acad. Sci. USA 114, E7479–E7488 (2017).
Dan, H., Ruan, T. & Sampogna, R. V. Circadian clock regulation of developmental time in the kidney. Cell Rep. 31, 107661 (2020).
Yamazaki, S. et al. Ontogeny of circadian organization in the rat. J. Biol. Rhythms 24, 55–63 (2009).
Ziv, L. & Gothilf, Y. Circadian time-keeping during early stages of development. Proc. Natl Acad. Sci. USA 103, 4146–4151 (2006).
Laranjeiro, R. & Whitmore, D. Transcription factors involved in retinogenesis are co-opted by the circadian clock following photoreceptor differentiation. Development 141, 2644–2656 (2014).
Zhao, J. et al. Drosophila clock can generate ectopic circadian clocks. Cell 113, 755–766 (2003).
Kilman, V. L. & Allada, R. Genetic analysis of ectopic circadian clock induction in Drosophila. J. Biol. Rhythms 24, 368–378 (2009).
Qu, M., Duffy, T., Hirota, T. & Kay, S. A. Nuclear receptor HNF4A transrepresses CLOCK:BMAL1 and modulates tissue-specific circadian networks. Proc. Natl Acad. Sci. USA 115, E12305–E12312 (2018).
Zhang, Y. et al. The microbiome stabilizes circadian rhythms in the gut. Proc. Natl Acad. Sci. USA 120, e2217532120 (2023).
Di Cara, F. & King-Jones, K. How clocks and hormones act in concert to control the timing of insect development. Curr. Top. Dev. Biol. 105, 1–36 (2013).
Rosselot, A. E. et al. Ontogeny and function of the circadian clock in intestinal organoids. Embo j. 41, e106973 (2022).
Yu, X. et al. TH17 cell differentiation is regulated by the circadian clock. Science 342, 727–730 (2013).
Alvarez-Dominguez, J. R. et al. Circadian entrainment triggers maturation of human in vitro islets. Cell Stem Cell 26, 108–122.e110 (2020).
Zhang, Z. B., Sinha, J., Bahrami-Nejad, Z. & Teruel, M. N. The circadian clock mediates daily bursts of cell differentiation by periodically restricting cell-differentiation commitment. Proc. Natl Acad. Sci. USA 119, e2204470119 (2022).
Boumard, B. & Bardin, A. J. An amuse-bouche of stem cell regulation: Underlying principles and mechanisms from adult Drosophila intestinal stem cells. Curr. Opin. Cell Biol. 73, 58–68 (2021).
Ma, Y., Chen, Z., Jin, Y. & Liu, W. Identification of a histone acetyltransferase as a novel regulator of Drosophila intestinal stem cells. FEBS Lett. 587, 1489–1495 (2013).
Jin, Y. et al. Brahma is essential for Drosophila intestinal stem cell proliferation and regulated by Hippo signaling. Elife 2, e00999 (2013).
Hemmers, S. & Rudensky, A. Y. The cell-intrinsic circadian clock is dispensable for lymphocyte differentiation and function. Cell Rep. 11, 1339–1349 (2015).
Bainbridge, S. P. & Bownes, M. Staging the metamorphosis of Drosophila melanogaster. Development 66, 57–80 (1981).
Bu B., Chen L., Zheng L., He W., Zhang L. Nipped‐A regulates the Drosophila circadian clock via histone deubiquitination. EMBO J. 39, e101259 (2020).
Benito, J., Houl, J. H., Roman, G. W. & Hardin, P. E. The blue-light photoreceptor CRYPTOCHROME is expressed in a subset of circadian oscillator neurons in the Drosophila CNS. J. Biol. Rhythms 23, 296–307 (2008).
Parasram, K., Bachetti, D., Carmona-Alcocer, V. & Karpowicz, P. Fluorescent reporters for studying circadian rhythms in Drosophila melanogaster. Methods Mol. Biol. 2482, 353–371 (2022).
Parsons, R., Parsons, R., Garner, N., Oster, H. & Rawashdeh, O. CircaCompare: a method to estimate and statistically support differences in mesor, amplitude and phase, between circadian rhythms. Bioinformatics 36, 1208–1212 (2019).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e3529 (2021).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Acknowledgements
We are grateful to the members of Karpowicz, Foley, and DeVeale labs and the technical staff at the Universities of Windsor and Alberta for their insightful advice and expertize. K.P., A.Z., and P.K. were funded by the Canada Foundation for Innovation, the Ontario Research Fund, and the Natural Sciences and Engineering Research Council of Canada (RGPIN-2020-04252 P.K.). B.D. was funded by the Natural Sciences and Engineering Research Council of Canada (RGPIN-2023-04028 BD). M.S., R.W., and E.F. were funded by Canadian Institutes of Health Research (MOP77746 EF). To the authors whose research we could not cite due to space limitations, we offer our apologies and thanks.
Author information
Authors and Affiliations
Contributions
Investigation: K.P., A.Z., M.S., and R.W.; Formal analysis: K.P., A.Z., R.W., and B.D.; Writing – Original Draft: K.P. and P.K.; Writing – Review & Editing: K.P., B.D., E.F., and P.K.; Conceptualization: P.K.; Supervision: E.F. and P.K.; Funding acquisition: E.F. and P.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks John Ewer and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parasram, K., Zuccato, A., Shin, M. et al. The emergence of circadian timekeeping in the intestine. Nat Commun 15, 1788 (2024). https://doi.org/10.1038/s41467-024-45942-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-45942-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.