Abstract
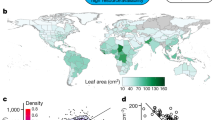
Early flowering plants are thought to have been woody species restricted to warm habitats1,2,3. This lineage has since radiated into almost every climate, with manifold growth forms4. As angiosperms spread and climate changed, they evolved mechanisms to cope with episodic freezing. To explore the evolution of traits underpinning the ability to persist in freezing conditions, we assembled a large species-level database of growth habit (woody or herbaceous; 49,064 species), as well as leaf phenology (evergreen or deciduous), diameter of hydraulic conduits (that is, xylem vessels and tracheids) and climate occupancies (exposure to freezing). To model the evolution of species’ traits and climate occupancies, we combined these data with an unparalleled dated molecular phylogeny (32,223 species) for land plants. Here we show that woody clades successfully moved into freezing-prone environments by either possessing transport networks of small safe conduits5 and/or shutting down hydraulic function by dropping leaves during freezing. Herbaceous species largely avoided freezing periods by senescing cheaply constructed aboveground tissue. Growth habit has long been considered labile6, but we find that growth habit was less labile than climate occupancy. Additionally, freezing environments were largely filled by lineages that had already become herbs or, when remaining woody, already had small conduits (that is, the trait evolved before the climate occupancy). By contrast, most deciduous woody lineages had an evolutionary shift to seasonally shedding their leaves only after exposure to freezing (that is, the climate occupancy evolved before the trait). For angiosperms to inhabit novel cold environments they had to gain new structural and functional trait solutions; our results suggest that many of these solutions were probably acquired before their foray into the cold.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
03 January 2014
The Dryad Digital Repository doi number has been updated.
References
Sinnott, E. W. & Bailey, I. W. The evolution of herbaceous plants and its bearing on certain problems of geology and climatology. J. Geol. 23, 289–306 (1915)
Wing, S. L. & Boucher, L. D. Ecological aspects of the Cretaceous flowering plant radiation. Annu. Rev. Earth Planet. Sci. 26, 379–421 (1998)
Feild, T. S., Arens, N. C., Doyle, J. A., Dawson, T. E. & Donoghue, M. J. Dark and disturbed: a new image of early angiosperm ecology. Paleobiology 30, 82–107 (2004)
Moles, A. T. et al. Global patterns in plant height. J. Ecol. 97, 923–932 (2009)
Tyree, M. T. & Zimmermann, M. H. Xylem Structure and the Ascent of Sap (Springer, 2002)
Cronquist, A. The Evolution and Classification of Flowering Plants. (Houghton Mifflin, 1968)
Kattge, J. et al. TRY—a global database of plant traits. Glob. Change Biol. 17, 2905–2935 (2011)
Stebbins, G. L. The probable growth habit of the earliest flowering plants. Ann. Mo. Bot. Gard. 52, 457–468 (1965)
Taylor, D. & Hickey, L. Phylogenetic evidence for the herbaceous origin of angiosperms. Plant Syst. Evol. 180, 137–156 (1992)
Soltis, D. E. et al. Angiosperm phylogeny: 17 genes, 640 taxa. Am. J. Bot. 98, 704–730 (2011)
Smith, S. A., Beaulieu, J. M. & Donoghue, M. J. An uncorrelated relaxed-clock analysis suggests an earlier origin for flowering plants. Proc. Natl Acad. Sci. USA 107, 5897–5902 (2010)
Spicer, R. & Groover, A. Evolution of development of vascular cambia and secondary growth. New Phytol. 186, 577–592 (2010)
Feild, T. S. & Wilson, J. P. Evolutionary voyage of angiosperm vessel structure-function and its significance for early angiosperm success. Int. J. Plant Sci. 173, 596–609 (2012)
Philippe, M. et al. Woody or not woody? Evidence for early angiosperm habit from the Early Cretaceous fossil wood record of Europe. Palaeoworld 17, 142–152 (2008)
Wiens, J. J. & Donoghue, M. J. Historical biogeography, ecology and species richness. Trends Ecol. Evol. 19, 639–644 (2004)
Donoghue, M. J. A phylogenetic perspective on the distribution of plant diversity. Proc. Natl Acad. Sci. USA 105, 11549–11555 (2008)
Wheeler, E. A., Baas, P. & Rodgers, S. Variations in dicot wood anatomy: a global analysis based on the Insidewood database. IAWA J. 28, 229–258 (2007)
Botta, A., Viovy, N., Ciais, P., Friedlingstein, P. & Monfray, P. A global prognostic scheme of leaf onset using satellite data. Glob. Change Biol. 6, 709–725 (2000)
Judd, W. S., Sanders, R. W. & Donoghue, M. J. Angiosperm family pairs: preliminary phylogenetic analysis. Harv. Pap. Bot. 5, 1–49 (1994)
Paton, A. J. et al. Towards target 1 of the global strategy for plant conservation: a working list of all known plant speciesprogress and prospects. Taxon 57, 602–611 (2008)
Loehle, C. Height growth rate tradeoffs determine northern and southern range limits for trees. J. Biogeogr. 25, 735–742 (1998)
Davis, S. D., Sperry, J. S. & Hacke, U. G. The relationship between xylem conduit diameter and cavitation caused by freezing. Am. J. Bot. 86, 1367–1372 (1999)
Maddison, W. P. Confounding asymmetries in evolutionary diversification and character change. Evolution 60, 1743–1746 (2006)
Soltis, D. E. et al. Phylogenetic relationships and character evolution analysis of Saxifragales using a supermatrix approach. Am. J. Bot. 100, 916–929 (2013)
Thomson, F. J., Moles, A. T., Auld, T. D. & Kingsford, R. T. Seed dispersal distance is more strongly correlated with plant height than with seed mass. J. Ecol. 99, 1299–1307 (2011)
Groover, A. T. What genes make a tree a tree? Trends Plant Sci. 10, 210–214 (2005)
Lens, F., Smets, E. & Melzer, S. Stem anatomy supports Arabidopsis thaliana as a model for insular woodiness. New Phytol. 193, 12–17 (2012)
Jansson, R., Rodríguez-Castañeda, G. & Harding, L. E. What can multiple phylogenies say about the latitudinal diversity gradient? A new look at the tropical conservatism, out-of-the-tropics and diversification rate hypotheses. Evolution 67, 1741–1755 (2013)
Beaulieu, J. M., O’Meara, B. C. & Donoghue, M. J. Identifying hidden rate changes in the evolution of a binary morphological character: the evolution of plant habit in campanulid angiosperms. Syst. Biol. 62, 725–737 (2013)
Acknowledgements
We thank T. Robertson and A. Hahn at the Global Biodiversity Information Facility for providing species’ georeference points, A. Ordonez for providing growth form data, and A. Miller and D. Ackerly for helpful comments on a draft of this manuscript. Support for this work was given to the working group “Tempo and Mode of Plant Trait Evolution: Synthesizing Data from Extant and Extinct Taxa” by the National Evolutionary Synthesis Center (NESCent), National Science Foundation grant #EF- 0905606 and Macquarie University Genes to Geoscience Research Centre.
Author information
Authors and Affiliations
Contributions
A.E.Z., W.K.C., D.C.T. and J.M.B. designed the initial project, wrote the original manuscript and carried out analyses. J.M.E., S.A.S. and D.C.T. constructed the timetree. J.M.E., R.G.F., D.J.M., B.C.O’M. and S.A.S. were major quantitative contributors, especially with the development of new methods, analyses, graphics and writing. A.T.M., P.B.R., D.L.R., D.E.S., P.F.S., I.J.W. and M.W. were large contributors through the development of initial ideas, methods, dataset curation, analyses and writing. L.A., R.I.B., A.C., R.G., F.H., M.R.L., J.O., P.S.S., N.G.S. and L.W. contributed data sets and discussions, and read drafts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Data and code are deposited at the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.63q27) and TRY (http://www.try-db.org/).
Extended data figures and tables
Extended Data Figure 1 Examples of the definition of ‘woody’.
a–d, We defined ‘woody’ as having a prominent aboveground stem that is persistent over time and with changing environmental conditions. a, Liriodendron tulipifera (Magnoliaceae), Joyce Kilmer Memorial Forest, Robbinsville, North Carolina, USA. b, Carnegiea giganteana (Cactaceae), Biosphere II, Tucson, Arizona, USA, c, Rhopalostylis sapida (Arecaceae) and Cyathea sp. (Cyatheaceae), Punakaiki, South Island, New Zealand. d, Pandanus sp. (Pandanaceae), Moreton Bay Research Station, North Stradbroke Island, Queensland, Australia (photographs by A.E.Z.).
Extended Data Figure 2 Reference timetree used for congruification analyses.
Results of the divergence time estimation of 639 taxa of seed plants from the reanalysis of a previously described10 phylogeny. Fossil calibrations are indicated at the nodes with green circles, and numbers correspond to fossils described in Supplementary Table 2. Concentric dashed circles represent 100-Myr intervals as indicated by the scale bar.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary References and Supplementary Tables 1-4. (PDF 2886 kb)
Rights and permissions
About this article
Cite this article
Zanne, A., Tank, D., Cornwell, W. et al. Three keys to the radiation of angiosperms into freezing environments. Nature 506, 89–92 (2014). https://doi.org/10.1038/nature12872
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12872
This article is cited by
-
Transnational conservation to anticipate future plant shifts in Europe
Nature Ecology & Evolution (2024)
-
Plant invasion and naturalization are influenced by genome size, ecology and economic use globally
Nature Communications (2024)
-
Fast height growth is key to non-native conifers invasiveness in temperate forests
Biological Invasions (2024)
-
Temperature dependence of carbon metabolism in the leaves in sun and shade in a subtropical forest
Oecologia (2024)
-
Linking plant nitrogen use efficiency with single traits, ecological strategies and phylogeny in a temperate steppe
Plant and Soil (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.