Abstract
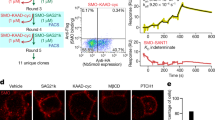
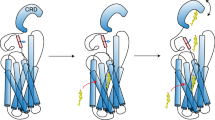
The smoothened (SMO) receptor, a key signal transducer in the hedgehog signalling pathway, is responsible for the maintenance of normal embryonic development and is implicated in carcinogenesis. It is classified as a class frizzled (class F) G-protein-coupled receptor (GPCR), although the canonical hedgehog signalling pathway involves the GLI transcription factors and the sequence similarity with class A GPCRs is less than 10%. Here we report the crystal structure of the transmembrane domain of the human SMO receptor bound to the small-molecule antagonist LY2940680 at 2.5 Å resolution. Although the SMO receptor shares the seven-transmembrane helical fold, most of the conserved motifs for class A GPCRs are absent, and the structure reveals an unusually complex arrangement of long extracellular loops stabilized by four disulphide bonds. The ligand binds at the extracellular end of the seven-transmembrane-helix bundle and forms extensive contacts with the loops.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ingham, P. W. & McMahon, A. P. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087 (2001)
Robbins, D. J., Fei, D. L. & Riobo, N. A. The Hedgehog signal transduction network. Sci. Signal. 5, re6 (2012)
Marigo, V., Davey, R. A., Zuo, Y., Cunningham, J. M. & Tabin, C. J. Biochemical evidence that patched is the Hedgehog receptor. Nature 384, 176–179 (1996)
Stone, D. M. et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 384, 129–134 (1996)
Taipale, J., Cooper, M. K., Maiti, T. & Beachy, P. A. Patched acts catalytically to suppress the activity of Smoothened. Nature 418, 892–896 (2002)
Corbit, K. C. et al. Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018–1021 (2005)
Klaus, A. & Birchmeier, W. Wnt signalling and its impact on development and cancer. Nature Rev. Cancer 8, 387–398 (2008)
Huang, H. C. & Klein, P. S. The Frizzled family: receptors for multiple signal transduction pathways. Genome Biol. 5, 234 (2004)
Schulte, G. International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors. Pharmacol. Rev. 62, 632–667 (2010)
Bhanot, P. et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382, 225–230 (1996)
Janda, C. Y., Waghray, D., Levin, A. M., Thomas, C. & Garcia, K. C. Structural basis of Wnt recognition by Frizzled. Science 337, 59–64 (2012)
Katritch, V., Cherezov, V. & Stevens, R. C. Structure-function of the G protein-coupled receptor superfamily. Annu Rev. Pharmacol. Toxicol. 53, 531–556 (2013)
Foord, S. M. et al. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol. Rev. 57, 279–288 (2005)
Ayers, K. L. & Therond, P. P. Evaluating Smoothened as a G-protein-coupled receptor for Hedgehog signalling. Trends Cell Biol. 20, 287–298 (2010)
Chen, W. et al. Activity-dependent internalization of Smoothened mediated by β-arrestin 2 and GRK2. Science 306, 2257–2260 (2004)
Riobo, N. A., Saucy, B., Dilizio, C. & Manning, D. R. Activation of heterotrimeric G proteins by Smoothened. Proc. Natl Acad. Sci. USA 103, 12607–12612 (2006)
Polizio, A. H. et al. Heterotrimeric Gi proteins link Hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. J. Biol. Chem. 286, 19589–19596 (2011)
Heretsch, P., Tzagkaroulaki, L. & Giannis, A. Modulators of the hedgehog signaling pathway. Bioorg. Med. Chem. 18, 6613–6624 (2010)
Bender, M. H. et al. Identification and characterization of a novel smoothened antagonist for the treatment of cancer with deregulated hedgehog signaling. Cancer Res. 71, 2819 (2011)
Hipskind, P. A., Patel, B. K. & Wilson, T. Disubstituted phthalazine hedgehog pathway antagonists. US patent 8,273,742 B2. (2010)
Cherezov, V. et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 (2007)
Wu, B. et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 330, 1066–1071 (2010)
Zhao, Y., Tong, C. & Jiang, J. Hedgehog regulates smoothened activity by inducing a conformational switch. Nature 450, 252–258 (2007)
Ballesteros, J. A. & Weinstein, H. in Methods in Neurosciences Vol. 25 (ed. Sealfon, S. C.) 366–428 (Academic, 1995)
Rasmussen, S. G. et al. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469, 175–180 (2011)
Yauch, R. L. et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science 326, 572–574 (2009)
Taipale, J. et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 406, 1005–1009 (2000)
Carroll, C. E., Marada, S., Stewart, D. P., Ouyang, J. X. & Ogden, S. K. The extracellular loops of Smoothened play a regulatory role in control of Hedgehog pathway activation. Development 139, 612–621 (2012)
Granier, S. et al. Structure of the δ-opioid receptor bound to naltrindole. Nature 485, 400–404 (2012)
Manglik, A. et al. Crystal structure of the μ-opioid receptor bound to a morphinan antagonist. Nature 485, 321–326 (2012)
Thompson, A. A. et al. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature 485, 395–399 (2012)
White, J. F. et al. Structure of the agonist-bound neurotensin receptor. Nature 490, 508–513 (2012)
Wu, H. et al. Structure of the human κ-opioid receptor in complex with JDTic. Nature 485, 327–332 (2012)
Zhang, C. et al. High-resolution crystal structure of human protease-activated receptor 1. Nature 492, 387–392 (2012)
Xie, J. et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391, 90–92 (1998)
Umbhauer, M. et al. The C-terminal cytoplasmic Lys-Thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/β-catenin signalling. EMBO J. 19, 4944–4954 (2000)
Wong, H. C. et al. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol. Cell 12, 1251–1260 (2003)
Krishnan, A., Almen, M. S., Fredriksson, R. & Schioth, H. B. The origin of GPCRs: identification of mammalian like Rhodopsin, Adhesion, Glutamate and Frizzled GPCRs in fungi. PLoS ONE 7, e29817 (2012)
Schwartz, T. W. & Rosenkilde, M. M. Is there a ‘lock’ for all agonist ‘keys’ in 7TM receptors? Trends Pharmacol. Sci. 17, 213–216 (1996)
Caffrey, M. & Cherezov, V. Crystallizing membrane proteins using lipidic mesophases. Nature Protocols 4, 706–731 (2009)
Cherezov, V., Peddi, A., Muthusubramaniam, L., Zheng, Y. F. & Caffrey, M. A robotic system for crystallizing membrane and soluble proteins in lipidic mesophases. Acta Crystallogr. D 60, 1795–1807 (2004)
Cherezov, V. et al. Rastering strategy for screening and centring of microcrystal samples of human membrane proteins with a sub-10 μm size X-ray synchrotron beam. J. R. Soc. Interface 6 (suppl. 5). S587–S597 (2009)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Terwilliger, T. C. et al. Decision-making in structure solution using Bayesian estimates of map quality: the PHENIX AutoSol wizard. Acta Crystallogr. D 65, 582–601 (2009)
Bricogne, G., Vonrhein, C., Flensburg, C., Schiltz, M. & Paciorek, W. Generation, representation and flow of phase information in structure determination: recent developments in and around SHARP 2. 0. Acta Crystallogr. D 59, 2023–2030 (2003)
Terwilliger, T. C. et al. Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr. D 64, 61–69 (2008)
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010)
Rominger, C. M. et al. Evidence for allosteric interactions of antagonist binding to the Smoothened receptor. J. Pharmacol. Exp. Ther. 329, 995–1005 (2009)
Acknowledgements
This work was supported by the National Institutes of Health Common Fund grant P50 GM073197 for technology development (V.C. and R.C.S.), PSI:Biology grant U54 GM094618 for biological studies and structure production (target GPCR-131) (V.K., V.C. and R.C.S.); F32 DK088392 (F.Y.S.); R01 MH61887, U19 MH82441, R01 DA27170 and the NIMH Psychoactive Drug Screening Program (X.-P.H. and B.L.R.) and the Michael Hooker Chair of Pharmacology (B.L.R.). We thank J. Velasquez for help with molecular biology, T. Trinh and M. Chu for help with baculovirus expression, K. Kadyshevskaya for assistance with figure preparation, A. Walker for assistance with manuscript preparation, D. Wacker for assistance with SAD data collection and processing and J. Smith, R. Fischetti and N. Sanishvili for assistance in development and use of the minibeam and beamtime at beamline 23-ID at the Advanced Photon Source, which is supported by National Cancer Institute grant Y1-CO-1020 and National Institute of General Medical Sciences grant Y1-GM-1104.
Author information
Authors and Affiliations
Contributions
C.W. designed and made the constructs, purified and crystallized the receptor in LCP, optimized crystallization conditions, grew crystals for data collection, assisted with crystal collection and heavy-atom soaking experiment, collected the diffraction data and prepared the manuscript. H.W. performed baculovirus expression of the receptor, purified the receptor, optimized crystallization conditions and prepared the manuscript. V.K. prepared the manuscript. G.W.H. solved and refined the structure and assisted with preparation of the manuscript. X.-P.H. performed the radioligand binding assay. W.L. assisted with data collection. F.Y.S. provided material and conditions for heavy-atom soaking experiment. B.L.R. supervised the radioligand binding experiment and assisted with preparing the manuscript. V.C. collected crystals, performed the heavy-atom soaking experiment, collected and processed diffraction data and assisted with preparation of the manuscript. R.C.S. was responsible for the overall project strategy and management and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Table 1, Supplementary Figures 1-11 and Supplementary references. (PDF 1934 kb)
Rights and permissions
About this article
Cite this article
Wang, C., Wu, H., Katritch, V. et al. Structure of the human smoothened receptor bound to an antitumour agent. Nature 497, 338–343 (2013). https://doi.org/10.1038/nature12167
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12167
This article is cited by
-
Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies
Signal Transduction and Targeted Therapy (2023)
-
Sonic Hedgehog Signaling Pathway: A Role in Pain Processing
Neurochemical Research (2023)
-
Universal platform for the generation of thermostabilized GPCRs that crystallize in LCP
Nature Protocols (2022)
-
Understanding Abnormal SMO-SHH Signaling in Autism Spectrum Disorder: Potential Drug Target and Therapeutic Goals
Cellular and Molecular Neurobiology (2022)
-
G protein-coupled receptors: structure- and function-based drug discovery
Signal Transduction and Targeted Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.