Abstract
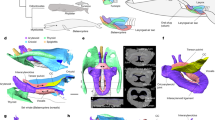
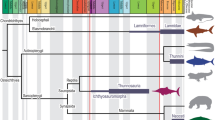
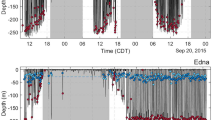
Top ocean predators have evolved multiple solutions to the challenges of feeding in the water1,2,3. At the largest scale, rorqual whales (Balaenopteridae) engulf and filter prey-laden water by lunge feeding4, a strategy that is unique among vertebrates1. Lunge feeding is facilitated by several morphological specializations, including bilaterally separate jaws that loosely articulate with the skull5,6, hyper-expandable throat pleats, or ventral groove blubber7, and a rigid y-shaped fibrocartilage structure branching from the chin into the ventral groove blubber8. The linkages and functional coordination among these features, however, remain poorly understood. Here we report the discovery of a sensory organ embedded within the fibrous symphysis between the unfused jaws that is present in several rorqual species, at both fetal and adult stages. Vascular and nervous tissue derived from the ancestral, anterior-most tooth socket insert into this organ, which contains connective tissue and papillae suspended in a gel-like matrix. These papillae show the hallmarks of a mechanoreceptor, containing nerves and encapsulated nerve termini. Histological, anatomical and kinematic evidence indicate that this sensory organ responds to both the dynamic rotation of the jaws during mouth opening and closure, and ventral groove blubber7 expansion through direct mechanical linkage with the y-shaped fibrocartilage structure. Along with vibrissae on the chin9, providing tactile prey sensation, this organ provides the necessary input to the brain for coordinating the initiation, modulation and end stages of engulfment, a paradigm that is consistent with unsteady hydrodynamic models and tag data from lunge-feeding rorquals10,11,12,13. Despite the antiquity of unfused jaws in baleen whales since the late Oligocene14 (∼23–28 million years ago), this organ represents an evolutionary novelty for rorquals, based on its absence in all other lineages of extant baleen whales. This innovation has a fundamental role in one of the most extreme feeding methods in aquatic vertebrates, which facilitated the evolution of the largest vertebrates ever.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sanderson, S. L. & Wassersug, R. in The Skull, Functional and Evolutionary Mechanisms Vol. 3 (eds Hanken, J. & Hall, B. K. ) 37–112 (Univ. Chicago Press, 1993)
Friedman, M. et al. 100-million-year dynasty of giant planktivorous bony fishes in the Mesozoic seas. Science 327, 990–993 (2010)
Werth, A. J. in Feeding: Form, Function and Evolution in Tetrapod Vertebrates (ed. Schwenk, K. ) 475–514 (Academic, 2000)
Goldbogen, J. A. The ultimate mouthful: lunge feeding in rorqual whales. Am. Sci. 98, 124–131 (2010)
Brodie, P. F. in Secondary Adaptation of Tetrapods to Life in Water (eds Mazin, J.-M. & de Buffrenil, V. ) 345–352 (Pfeil, 2001)
Lambertsen, R., Ulrich, N. & Straley, J. Frontomandibular stay of Balaenopteridae: a mechanism for momentum recapture during feeding. J. Mamm. 76, 877–899 (1995)
Orton, L. S. & Brodie, P. F. Engulfing mechanics of fin whales. Can. J. Zool. 65, 2898–2907 (1987)
Pivorunas, A. Fibrocartilage skeleton and related structures of the ventral pouch of balaenopterid whales. J. Morphol. 151, 299–313 (1977)
Ling, J. K. in Functional Anatomy of Marine Mammals (ed. Harrison, R. J. ) 387–415 (Academic, 1977)
Potvin, J., Goldbogen, J. A. & Shadwick, R. E. Passive versus active engulfment: verdict from trajectory simulations of lunge-feeding fin whales. J. R. Soc. Interface 6, 1005–1025 (2009)
Potvin, J., Goldbogen, J. A. & Shadwick, R. E. Scaling of lunge feeding in rorqual whales: an integrated model of engulfment duration. J. Theor. Biol. 267, 437–453 (2010)
Goldbogen, J. A. et al. Mechanics, hydrodynamics and energetics of blue whale lunge feeding: efficiency dependence on krill density. J. Exp. Biol. 214, 131–146 (2011)
Goldbogen, J. A. et al. Scaling of lunge feeding performance in rorqual whales: mass-specific energy expenditure increases with body size and progressively limits diving capacity. Funct. Ecol. 26, 216–226 (2012)
Fitzgerald, E. M. G. Archaeocete-like jaws in a baleen whale. Biol. Lett. 8, 94–96 (2012)
Deméré, T. A., McGowen, M. R., Berta, A. & Gatesy, J. Morphological and molecular evidence for a step-wise evolutionary transition from teeth to baleen in mysticete whales. Syst. Biol. 57, 15–37 (2008)
Fitzgerald, E. M. G. The morphology and systematics of Mammalodon colliveri (Cetacea: Mysticeti), a toothed mysticete from the Oligocene of Australia. Zool. J. Linn. Soc. 158, 367–476 (2010)
Goldbogen, J. A., Pyenson, N. D. & Shadwick, R. E. Big gulps require high drag for fin whale lunge-feeding. Mar. Ecol. Prog. Ser. 349, 289–301 (2007)
Lambertsen, R. H. Internal mechanism of rorqual feeding. J. Mamm. 64, 76–88 (1983)
Brodie, P. F. Noise generated by the jaw actions of feeding fin whales. Can. J. Zool. 71, 2546–2550 (1993)
Currey, J. D. Bones: Structure and Mechanics (Princeton Univ. Press, 2002)
Best, R. C. The tusk of the narwhal (Monodon monoceros L.): interpretation of its function (Mammalia: Cetacea). Can. J. Zool. 59, 2386–2393 (1981)
McKenna, M. F., Cranford, T. W., Berta, A. & Pyenson, N. D. Morphological diversity of the odontocete melon: implications for acoustic function. Mar. Mamm. Sci. (2011)
Tershy, B. R. & Wiley, D. M. Asymmetrical pigmentation in the fin whale: a test of two feeding related hypotheses. Mar. Mamm. Sci. 8, 315–318 (1992)
Canning, C. et al. Population-level lateralized feeding behaviour in North Atlantic humpback whales, Megaptera novaeangliae . Anim. Behav. 82, 901–909 (2011)
Catania, K. C., Hare, J. F. & Campbell, K. L. Water shrews detect movement, shape, and smell to find prey underwater. Proc. Natl Acad. Sci. USA 105, 571–576 (2008)
Czech-Damal, N. U. et al. Electroreception in the Guiana dolphin (Sotalia guianensis). Proc. R. Soc. Lond. B 279, 663–668 (2012)
Thewissen, J. G. M. & Nummela, S. Sensory Evolution on the Threshold: Adaptations in Secondarily Aquatic Vertebrates. (Univ. California Press, 2008)
Scapino, R. Morphological investigation into functions of the jaw symphysis in carnivorans. J. Morphol. 167, 339–375 (1981)
Johnston, C. et al. Observations on the musculoskeletal anatomy of the head of a neonate gray whale (Eschrichtius robustus). Mar. Mamm. Sci. 26, 186–194 (2010)
Reeves, R. R. & Smith, T. D. in Whales, Whaling, and Ocean Ecosystems (eds Estes, J. A. et al.) 82–101 (Univ. California Press, 2006)
Acknowledgements
For logistical support, we thank K. Loftsson and the staff at Hvalur hf; D. Ólafsdóttir, S. D. Halldórsson and G. A. Víkingsson at the Marine Research Institute, Reykjavík; G. Bergmann and the staff at Hrefnuveiðimenn ehf; S. Raverty; P. F. Brodie; and A. Trites. For additional samples and data, we also thank P.-Y. Daoust, G. Williams, the Amarok Hunters and Trappers Organization of Iqaluit, Captain S. Awa and the Inuit whaling crew from Iqaluit, J. Higgins and the Cascadia Research Collective, M. R. Buono, A. van Helden and the Museum of New Zealand Te Papa Tongarewa, and R. E. Fordyce. We also thank T. S. Hunter for assistance with laboratory samples. Comments from D. J. Bohaska, M. T. Carrano, R. B. Irmis, J. G. Mead, J. F. Parham, C. W. Potter and J. Velez-Juarbe improved this manuscript. N.D.P. was supported by a postdoctoral research fellowship from the Natural Sciences and Engineering Research Council of Canada and by funding from the Smithsonian Institution and its Remington Kellogg Fund. J.A.G. was supported by a University Graduate Fellowship for Research from the University of British Columbia, a Scripps Postdoctoral Research Fellowship and NSERC funding to R.E.S.
Author information
Authors and Affiliations
Contributions
N.D.P., J.A.G., A.W.V. and R.E.S. conducted field and laboratory investigations and wrote the paper. G.S. provided expertise with XRCT scanning and image reconstruction, and R.L.D. supervised the MRI reconstructions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text, Supplementary Table 1, Supplementary Figures 1- 9 and Supplementary References. (PDF 6605 kb)
Supplementary Movie 1
This movie shows a mandibular symphysis of an adult fin whale (B. physalus), rendered in 3D. (MOV 19914 kb)
Rights and permissions
About this article
Cite this article
Pyenson, N., Goldbogen, J., Vogl, A. et al. Discovery of a sensory organ that coordinates lunge feeding in rorqual whales. Nature 485, 498–501 (2012). https://doi.org/10.1038/nature11135
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11135
This article is cited by
-
First filter feeding in the Early Triassic: cranial morphological convergence between Hupehsuchus and baleen whales
BMC Ecology and Evolution (2023)
-
Minke whale feeding rate limitations suggest constraints on the minimum body size for engulfment filtration feeding
Nature Ecology & Evolution (2023)
-
Anatomical, Ontogenetic, and Genomic Homologies Guide Reconstructions of the Teeth-to-Baleen Transition in Mysticete Whales
Journal of Mammalian Evolution (2022)
-
Magnetic resonance imaging and computed tomography as tools for the investigation of sperm whale (Physeter macrocephalus) teeth and eye
Acta Veterinaria Scandinavica (2017)
-
Evidence for the perceptual origin of right-sided feeding biases in cetaceans
Animal Cognition (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.