Abstract
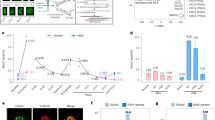
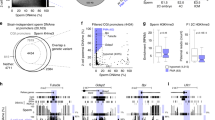
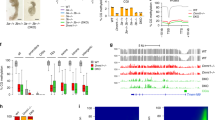
DNA methylation is highly dynamic during mammalian embryogenesis. It is broadly accepted that the paternal genome is actively depleted of 5-methylcytosine at fertilization, followed by passive loss that reaches a minimum at the blastocyst stage. However, this model is based on limited data, and so far no base-resolution maps exist to support and refine it. Here we generate genome-scale DNA methylation maps in mouse gametes and from the zygote through post-implantation. We find that the oocyte already exhibits global hypomethylation, particularly at specific families of long interspersed element 1 and long terminal repeat retroelements, which are disparately methylated between gametes and have lower methylation values in the zygote than in sperm. Surprisingly, the oocyte contributes a unique set of differentially methylated regions (DMRs)—including many CpG island promoters—that are maintained in the early embryo but are lost upon specification and absent from somatic cells. In contrast, sperm-contributed DMRs are largely intergenic and become hypermethylated after the blastocyst stage. Our data provide a genome-scale, base-resolution timeline of DNA methylation in the pre-specified embryo, when this epigenetic modification is most dynamic, before returning to the canonical somatic pattern.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 16, 6–21 (2002)
Jaenisch, R. & Bird, A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genet. 33 (Suppl.). 245–254 (2003)
Suzuki, M. M. & Bird, A. DNA methylation landscapes: provocative insights from epigenomics. Nature Rev. Genet. 9, 465–476 (2008)
Meissner, A. et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 454, 766–770 (2008)
Weber, M. et al. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nature Genet. 37, 853–862 (2005)
Weber, M. et al. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nature Genet. 39, 457–466 (2007)
Ji, H. et al. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature 467, 338–342 (2010)
Wossidlo, M. et al. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nature Commun. 2, 241 (2011)
Gu, T. P. et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature 477, 606–610 (2011)
Inoue, A. & Zhang, Y. Replication-dependent loss of 5-hydroxymethylcytosine in mouse preimplantation embryos. Science 334, 194 (2011)
Reik, W., Dean, W. & Walter, J. Epigenetic reprogramming in mammalian development. Science 293, 1089–1093 (2001)
Kafri, T. et al. Developmental pattern of gene-specific DNA methylation in the mouse embryo and germ line. Genes Dev. 6, 705–714 (1992)
Borgel, J. et al. Targets and dynamics of promoter DNA methylation during early mouse development. Nature Genet. 42, 1093–1100 (2010)
Meissner, A. Epigenetic modifications in pluripotent and differentiated cells. Nature Biotechnol. 28, 1079–1088 (2010)
Razin, A. & Shemer, R. DNA methylation in early development. Hum. Mol. Genet. 4, 1751–1755 (1995)
Monk, M., Boubelik, M. & Lehnert, S. Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development 99, 371–382 (1987)
Rougier, N. et al. Chromosome methylation patterns during mammalian preimplantation development. Genes Dev. 12, 2108–2113 (1998)
Mayer, W., Niveleau, A., Walter, J., Fundele, R. & Haaf, T. Demethylation of the zygotic paternal genome. Nature 403, 501–502 (2000)
Lane, N. et al. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis 35, 88–93 (2003)
Oswald, J. et al. Active demethylation of the paternal genome in the mouse zygote. Curr. Biol. 10, 475–478 (2000)
Santos, F., Hendrich, B., Reik, W. & Dean, W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev. Biol. 241, 172–182 (2002)
Kim, S. H. et al. Differential DNA methylation reprogramming of various repetitive sequences in mouse preimplantation embryos. Biochem. Biophys. Res. Commun. 324, 58–63 (2004)
Bock, C. et al. Quantitative comparison of genome-wide DNA methylation mapping technologies. Nature Biotechnol. 28, 1106–1114 (2010)
Harris, R. A. et al. Comparison of sequencing-based methods to profile DNA methylation and identification of monoallelic epigenetic modifications. Nature Biotechnol. 28, 1097–1105 (2010)
Smallwood, S. A. et al. Dynamic CpG island methylation landscape in oocytes and preimplantation embryos. Nature Genet. 43, 811–814 (2011)
Davis, T. & Vaisvila, R. High sensitivity 5-hydroxymethylcytosine detection in Balb/C brain tissue. J. Vis. Exp. 48, (2011)
Ficz, G. et al. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature 473, 398–402 (2011)
Szulwach, K. E. et al. Integrating 5-hydroxymethylcytosine into the epigenomic landscape of human embryonic stem cells. PLoS Genet. 7, e1002154 (2011)
Williams, K. et al. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature 473, 343–348 (2011)
Wu, H. et al. Genome-wide analysis of 5-hydroxymethylcytosine distribution reveals its dual function in transcriptional regulation in mouse embryonic stem cells. Genes Dev. 25, 679–684 (2011)
Xu, Y. et al. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol. Cell 42, 451–464 (2011)
Branco, M. R., Ficz, G. & Reik, W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nature Rev. Genet. 13, 7–13 (2011)
Santos, F., Hendrich, B., Reik, W. & Dean, W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev. Biol. 241, 172–182 (2002)
Hajkova, P. et al. Genome-wide reprogramming in the mouse germ line entails the base excision repair pathway. Science 329, 78–82 (2010)
Wossidlo, M. et al. Dynamic link of DNA demethylation, DNA strand breaks and repair in mouse zygotes. EMBO J. 29, 1877–1888 (2010)
Popp, C. et al. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature 463, 1101–1105 (2010)
Mouse Genome Sequencing Consortium Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562 (2002)
Ichiyanagi, K. et al. Locus- and domain-dependent control of DNA methylation at mouse B1 retrotransposons during male germ cell development. Genome Res. 21, 2058–2066 (2011)
Goodier, J. L., Ostertag, E. M., Du, K. & Kazazian, H. H., Jr A novel active L1 retrotransposon subfamily in the mouse. Genome Res. 11, 1677–1685 (2001)
Edwards, C. A. & Ferguson-Smith, A. Mechanisms regulating imprinted genes in clusters. Curr. Opin. Cell Biol. 19, 281–289 (2007)
Bestor, T. H. The DNA methyltransferases of mammals. Hum. Mol. Genet. 9, 2395–2402 (2000)
Hirasawa, R. et al. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev. 22, 1607–1616 (2008)
Lucifero, D. et al. Coordinate regulation of DNA methyltransferase expression during oogenesis. BMC Dev. Biol. 7, 36 (2007)
Ramsahoye, B. H. et al. Non-CpG methylation is prevalent in embryonic stem cells and may be mediated by DNA methyltransferase 3a. Proc. Natl Acad. Sci. USA 97, 5237–5242 (2000)
Ziller, M. J. et al. Genomic distribution and inter-sample variation of non-CpG methylation across human cell types. PLoS Genet. 7, e1002389 (2011)
Haines, T., Rodenhiser, D. & Ainsworth, P. Allele-specific non-CpG methylation of the Nf1 gene during early mouse development. Dev. Biol. 240, 585–598 (2001)
Tomizawa, S. et al. Dynamic stage-specific changes in imprinted differentially methylated regions during early mammalian development and prevalence of non-CpG methylation in oocytes. Development 138, 811–820 (2011)
Beraldi, R., Pittoggi, C., Sciamanna, I., Mattei, E. & Spadafora, C. Expression of LINE-1 retroposons is essential for murine preimplantation development. Mol. Reprod. Dev. 73, 279–287 (2006)
Kigami, D., Minami, N., Takayama, H. & Imai, H. MuERV-L is one of the earliest transcribed genes in mouse one-cell embryos. Biol. Reprod. 68, 651–654 (2003)
Okada, Y., Yamagata, K., Hong, K., Wakayama, T. & Zhang, Y. A role for the elongator complex in zygotic paternal genome demethylation. Nature 463, 554–558 (2010)
Nagy, A. Manipulating the Mouse Embryo: a Laboratory Manual 3rd edn (Cold Spring Harbor Laboratory Press, 2003)
Smith, Z. D., Gu, H., Bock, C., Gnirke, A. & Meissner, A. High-throughput bisulfite sequencing in mammalian genomes. Methods 48, 226–232 (2009)
Gu, H. et al. Preparation of reduced representation bisulfite sequencing libraries for genome-scale DNA methylation profiling. Nature Protocols 6, 468–481 (2011)
Gu, H. et al. Genome-scale DNA methylation mapping of clinical samples at single-nucleotide resolution. Nature Methods 7, 133–136 (2010)
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995)
Blake, J. A., Bult, C. J., Kadin, J. A., Richardson, J. E. & Eppig, J. T. The Mouse Genome Database (MGD): premier model organism resource for mammalian genomics and genetics. Nucleic Acids Res. 39, D842–D848 (2011)
Acknowledgements
We would like to thank all members of the Meissner and Regev laboratories. M. Garber, N. Yosef, J. Ye, R. Koche, C. Bock, R. Maehr and D. Egli for technical advice and discussion. We thank all members of the Broad Sequencing Platform, in particular F. Kelly and J. Meldrim, T. Fennel, K. Tibbetts and J. Fostel. We also thank S. Levine, M. Gravina and K. Thai from the MIT BioMicro Center. A.R. is an investigator of the Merkin Foundation for Stem Cell Research at the Broad Institute. This work was supported by the NIH Pioneer Award (5DP1OD003958), the Burroughs Wellcome Career Award at the Scientific Interface and HHMI (to A.R.), the Harvard Stem Cell Institute (to T.S.M.) and the NIH (5RC1AA019317, U01ES017155 and P01GM099117), the Massachusetts Life Science Center and the Pew Charitable Trusts (to A.M.) and a Center for Excellence in Genome Science from the NHGRI (1P50HG006193-01, to A.R. and A.M.).
Author information
Authors and Affiliations
Contributions
Z.D.S. and A.M. conceived the study and Z.D.S., M.M.C. and A.M. facilitated its design. Z.D.S. collected samples and performed methylation profiling, M.M.C. performed all analysis with assistance from T.S.M. and Z.D.S. H.G. and A.G. provided critical technical assistance and expertise. Z.D.S., M.M.C., T.S.M., A.R. and A.M. interpreted the data. Z.D.S., M.M.C. and A.M. wrote the paper with the assistance of the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-11 and full legends for Supplementary Tables 1- 4 and Supplementary Movie 1. (PDF 1636 kb)
Supplementary Table 1
This file contains promoter methylation levels at fertilization and across early embryonic development - see legend in Supplementary Information file. (XLS 3760 kb)
Supplementary Table 2
This file contains Long Interspersed Element (LINE) retrotransposon feature methylation across early embryonic development - see legend in Supplementary Information file. (XLS 28 kb)
Supplementary Table 3
This file contains Long Terminal Repeat (LTR) retrotransposon feature methylation across early embryonic development - see legend in Supplementary Information file. (XLS 42 kb)
Supplementary Table 4
This file contains feature designation and methylation status for identified oocyte-contributed Differentially Methylated Regions (DMRs) across early embryonic development - see legend in Supplementary Information file. (XLS 149 kb)
Supplementary Movie 1
This file contains a movie of a representative polar body biopsy for zygotes and cleavage stage embryos collected in this study - see legend in Supplementary Information file. (MOV 7166 kb)
Rights and permissions
About this article
Cite this article
Smith, Z., Chan, M., Mikkelsen, T. et al. A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature 484, 339–344 (2012). https://doi.org/10.1038/nature10960
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10960
This article is cited by
-
PRAMEL7 and CUL2 decrease NuRD stability to establish ground-state pluripotency
EMBO Reports (2024)
-
Epigenetic changes in sperm are associated with paternal and child quantitative autistic traits in an autism-enriched cohort
Molecular Psychiatry (2024)
-
Extensive DNA methylome rearrangement during early lamprey embryogenesis
Nature Communications (2024)
-
Paternal DNA methylation is remodeled to maternal levels in rice zygote
Nature Communications (2023)
-
Regulation, functions and transmission of bivalent chromatin during mammalian development
Nature Reviews Molecular Cell Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.