Abstract
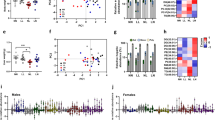
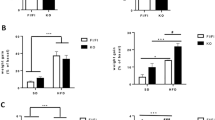
The global prevalence of obesity is increasing across most ages in both sexes. This is contributing to the early emergence of type 2 diabetes and its related epidemic1,2. Having either parent obese is an independent risk factor for childhood obesity3. Although the detrimental impacts of diet-induced maternal obesity on adiposity and metabolism in offspring are well established4, the extent of any contribution of obese fathers is unclear, particularly the role of non-genetic factors in the causal pathway. Here we show that paternal high-fat-diet (HFD) exposure programs β-cell ‘dysfunction’ in rat F1 female offspring. Chronic HFD consumption in Sprague–Dawley fathers induced increased body weight, adiposity, impaired glucose tolerance and insulin sensitivity. Relative to controls, their female offspring had an early onset of impaired insulin secretion and glucose tolerance that worsened with time, and normal adiposity. Paternal HFD altered the expression of 642 pancreatic islet genes in adult female offspring (P < 0.01); genes belonged to 13 functional clusters, including cation and ATP binding, cytoskeleton and intracellular transport. Broader pathway analysis of 2,492 genes differentially expressed (P < 0.05) demonstrated involvement of calcium-, MAPK- and Wnt-signalling pathways, apoptosis and the cell cycle. Hypomethylation of the Il13ra2 gene, which showed the highest fold difference in expression (1.76-fold increase), was demonstrated. This is the first report in mammals of non-genetic, intergenerational transmission of metabolic sequelae of a HFD from father to offspring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
Primary accessions
Gene Expression Omnibus
Data deposits
Gene expression data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo) and are accessible using GEO series accession number GSE19877.
References
Wang, Y. & Lobstein, T. Worldwide trends in childhood overweight and obesity. Int. J. Pediatr. Obes. 1, 11–25 (2006)
Pinhas-Hamiel, O. & Zeitler, P. The global spread of type 2 diabetes mellitus in children and adolescents. J. Pediatr. 146, 693–700 (2005)
Whitaker, R. C., Wright, J. A., Pepe, M. S., Seidel, K. D. & Dietz, W. H. Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 337, 869–873 (1997)
Morris, M. Early life influences on obesity risk: maternal overnutrition and programming of obesity. Expert Rev. Endocrinol. Metab. 4, 625–637 (2009)
Tarquini, B., Tarquini, R., Perfetto, F., Cornelissen, G. & Halberg, F. Genetic and environmental influences on human cord blood leptin concentration. Pediatrics 103, 998–1006 (1999)
Power, C., Li, L., Manor, O. & Davey Smith, G. Combination of low birth weight and high adult body mass index: at what age is it established and what are its determinants? J. Epidemiol. Community Health 57, 969–973 (2003)
Bouchard, C. Childhood obesity: are genetic differences involved? Am. J. Clin. Nutr. 89, 1494S–1501S (2009)
Guo, Y. F. et al. Assessment of genetic linkage and parent-of-origin effects on obesity. J. Clin. Endocrinol. Metab. 91, 4001–4005 (2006)
Le Stunff, C., Fallin, D. & Bougneres, P. Paternal transmission of the very common class I INS VNTR alleles predisposes to childhood obesity. Nature Genet. 29, 96–99 (2001)
Gluckman, P. D. et al. Towards a new developmental synthesis: adaptive developmental plasticity and human disease. Lancet 373, 1654–1657 (2009)
Li, L., Law, C., Lo Conte, R. & Power, C. Intergenerational influences on childhood body mass index: the effect of parental body mass index trajectories. Am. J. Clin. Nutr. 89, 551–557 (2009)
Dunn, G. A. & Bale, T. L. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 150, 4999–5009 (2009)
Ghanayem, B. I., Bai, R., Kissling, G. E., Travlos, G. & Hoffler, U. Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol. Reprod. 82, 96–104 (2009)
Kasturi, S. S., Tannir, J. & Brannigan, R. E. The metabolic syndrome and male infertility. J. Androl. 29, 251–259 (2008)
Figueroa-Colon, R., Arani, R. B., Goran, M. I. & Weinsier, R. L. Paternal body fat is a longitudinal predictor of changes in body fat in premenarcheal girls. Am. J. Clin. Nutr. 71, 829–834 (2000)
Leibel, N. I., Baumann, E. E., Kocherginsky, M. & Rosenfield, R. L. Relationship of adolescent polycystic ovary syndrome to parental metabolic syndrome. J. Clin. Endocrinol. Metab. 91, 1275–1283 (2006)
Jimenez-Chillaron, J. C. et al. Intergenerational transmission of glucose intolerance and obesity by in utero undernutrition in mice. Diabetes 58, 460–468 (2009)
Sone, H. & Kagawa, Y. Pancreatic β cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 48, 58–67 (2005)
Henquin, J. C., Nenquin, M., Ravier, M. A. & Szollosi, A. Shortcomings of current models of glucose-induced insulin secretion. Diabetes Obes. Metab. 11 (suppl. 4). 168–179 (2009)
Wang, Z. & Thurmond, D. C. Mechanisms of biphasic insulin-granule exocytosis—roles of the cytoskeleton, small GTPases and SNARE proteins. J. Cell Sci. 122, 893–903 (2009)
Fujisawa, T., Joshi, B., Nakajima, A. & Puri, R. K. A novel role of interleukin-13 receptor α2 in pancreatic cancer invasion and metastasis. Cancer Res. 69, 8678–8685 (2009)
David, M., Bertoglio, J. & Pierre, J. TNF-α potentiates IL-4/IL-13-induced IL-13Rα2 expression. Ann. NY Acad. Sci. 973, 207–209 (2002)
Zhang, X. Y. et al. The major histocompatibility complex class II promoter-binding protein RFX (NF-X) is a methylated DNA-binding protein. Mol. Cell. Biol. 13, 6810–6818 (1993)
Lindsay, R. S. et al. Type 2 diabetes and low birth weight: the role of paternal inheritance in the association of low birth weight and diabetes. Diabetes 49, 445–449 (2000)
Hypponen, E., Smith, G. D. & Power, C. Parental diabetes and birth weight of offspring: intergenerational cohort study. Br. Med. J. 326, 19–20 (2003)
Hattersley, A. T. & Tooke, J. E. The fetal insulin hypothesis: an alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet 353, 1789–1792 (1999)
Sharpe, R. M. Environmental/lifestyle effects on spermatogenesis. Phil. Trans. R. Soc. Lond. B 365, 1697–1712 (2010)
Robertson, S. A. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 322, 43–52 (2005)
Aitken, R. J., Koopman, P. & Lewis, S. E. Seeds of concern. Nature 432, 48–52 (2004)
Du Plessis, S. S., Cabler, S., McAlister, D. A., Sabanegh, E. & Agarwal, A. The effect of obesity on sperm disorders and male infertility. Nature Rev. Urol. 7, 153–161 (2010)
Prior, L. J., Velkoska, E., Watts, R., Cameron-Smith, D. & Morris, M. J. Undernutrition during suckling in rats elevates plasma adiponectin and its receptor in skeletal muscle regardless of diet composition: a protective effect? Int. J. Obes. (Lond) 32, 1585–1594 (2008)
Chamson-Reig, A., Thyssen, S. M., Arany, E. & Hill, D. J. Altered pancreatic morphology in the offspring of pregnant rats given reduced dietary protein is time and gender specific. J. Endocrinol. 191, 83–92 (2006)
Lacy, P. E. & Kostianovsky, M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16, 35–39 (1967)
Laybutt, D. R. et al. Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to β-cell survival during chronic hyperglycemia. Diabetes 51, 413–423 (2002)
Laybutt, D. R. et al. Critical reduction in β-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J. Biol. Chem. 278, 2997–3005 (2003)
Irizarry, R. A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003)
Huang, da W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols 4, 44–57 (2009)
Dennis, G., Jr et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 4, P3 (2003)
Saeed, A. I. et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34, 374–378 (2003)
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000)
Olek, A., Oswald, J. & Walter, J. A modified and improved method for bisulphite based cytosine methylation analysis. Nucleic Acids Res. 24, 5064–5066 (1996)
Grunau, C., Schattevoy, R., Mache, N. & Rosenthal, A. MethTools—a toolbox to visualize and analyze DNA methylation data. Nucleic Acids Res. 28, 1053–1058 (2000)
Acknowledgements
This work is supported by the National Health and Medical Research Council (NHMRC) of Australia (M.J.M.). S.F.N. is supported by Ministry of Higher Education and National University of Malaysia, R.C.Y.L. is supported by the NHMRC Peter Doherty Fellowship.
Author information
Authors and Affiliations
Contributions
S.F.N. and M.J.M. designed the study. S.F.N. performed animal work, histology, islet harvest and RNA extraction, data analysis and wrote the manuscript. M.J.M. supervised the project and wrote the manuscript. R.C.Y.L. conducted microarray data analysis. D.R.L. assisted with islet harvest. R.B. conducted bisulphite sequencing and DNA methylation analysis. J.A.O. conducted ingenuity analysis and wrote the manuscript. All authors contributed to data interpretation, reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-4 and Supplementary Figures 1-4 with legends. (PDF 6792 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Ng, SF., Lin, R., Laybutt, D. et al. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 467, 963–966 (2010). https://doi.org/10.1038/nature09491
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09491
This article is cited by
-
Dysfunction of DMT1 and miR-135b in the gut-testis axis in high-fat diet male mice
Genes & Nutrition (2024)
-
Determinants of obesity in Latin America
Nature Metabolism (2024)
-
Parental obesity predisposes to exacerbated metabolic and inflammatory disturbances in childhood obesity within the framework of an altered profile of trace elements
Nutrition & Diabetes (2024)
-
Inheritance of perturbed methylation and metabolism caused by uterine malnutrition via oocytes
BMC Biology (2023)
-
Paternal high-fat diet altered SETD2 gene methylation in sperm of F0 and F1 mice
Genes & Nutrition (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.