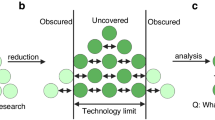
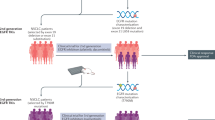
Abstract
The emphasis in cancer drug development has shifted from cytotoxic, non-specific chemotherapies to molecularly targeted, rationally designed drugs promising greater efficacy and less side effects. Nevertheless, despite some successes drug development remains painfully slow. Here, we highlight the issues involved and suggest ways in which this process can be improved and expedited. We envision an increasing shift to integrated cancer research and biomarker-driven adaptive and hypothesis testing clinical trials. The goal is the development of specific cancer medicines to treat the individual patient, with treatment selection being driven by a detailed understanding of the genetics and biology of the patient and their cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Workman, P. & de Bono, J. Targeted therapeutics for cancer treatment: major progress towards personalised molecular medicine. Curr. Opin. Pharmacol. 8, 359–362 (2008)
Carden, C. P., Banerji, U., Kaye, S. B., Workman, P. & de Bono, J. S. From darkness to light with biomarkers in early clinical trials of cancer drugs. Clin. Pharmacol. Ther. 85, 131–133 (2009)
DiMasi, J. A. & Grabowski, H. G. Economics of new oncology drug development. J. Clin. Oncol. 25, 209–216 (2007)
Carden, C. P. et al. Can molecular biomarker-based patient selection in Phase I trials accelerate anticancer drug development? Drug Discov. Today 15, 88–97 (2009)
Betensky, R. A., Louis, D. N. & Cairncross, J. G. Influence of unrecognized molecular heterogeneity on randomized clinical trials. J. Clin. Oncol. 20, 2495–2499 (2002)
Sobrero, A. & Bruzzi, P. Incremental advance or seismic shift? The need to raise the bar of efficacy for drug approval. J. Clin. Oncol. 27, 5868–5873 (2009)
Collins, I. & Workman, P. New approaches to molecular cancer therapeutics. Nature Chem. Biol. 2, 689–700 (2006)
de Bono, J. S., Tolcher, A. W. & Rowinsky, E. K. The future of cytotoxic therapy: selective cytotoxicity based on biology is the key. Breast Cancer Res. 5, 154–159 (2003)
Sawyers, C. L. The cancer biomarker problem. Nature 452, 548–552 (2008)A thoughtful discussion of the cancer biomarker problem.
Weinstein, I. B. Cancer. addiction to oncogenes–the Achilles heal of cancer. Science 297, 63–64 (2002)
Luo, J., Solimini, N. L. & Elledge, S. J. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136, 823–837 (2009)An important review updating the hallmarks of cancer originally proposed by Hanahan and Weinberg (ref. 14) and summarising new approaches to cancer therapy.
Ashworth, A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J. Clin. Oncol. 26, 3785–3790 (2008)
Kaelin, W. G., Jr The concept of synthetic lethality in the context of anticancer therapy. Nature Rev. Cancer 5, 689–698 (2005)
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000)
Iorns, E., Lord, C. J., Turner, N. & Ashworth, A. Utilizing RNA interference to enhance cancer drug discovery. Nature Rev. Drug Discov. 6, 556–568 (2007)
Hennessy, B. T., Gonzalez-Angulo, A. M., Carey, M. S. & Mills, G. B. A systems approach to analysis of molecular complexity in breast cancer. Clin. Cancer Res. 15, 417–419 (2009)
Kreeger, P. K. & Lauffenburger, D. A. Cancer systems biology: a network modeling perspective. Carcinogenesis 31, 2–8 (2010)
Becher, O. J. & Holland, E. C. Genetically engineered models have advantages over xenografts for preclinical studies. Cancer Res. 66, 3355–3359 (2006)
Sausville, E. A. & Burger, A. M. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 66, 3351–3354,–discussion 3354 (2006)
Lord, C. J. & Ashworth, A. Biology-driven cancer drug development: back to the future. BMC Biol. 8, 38 (2010)
Goodsaid, F. & Frueh, F. Biomarker qualification pilot process at the US Food and Drug Administration. AAPS J. 9, E105–E108 (2007)
Workman, P. et al. Minimally invasive pharmacokinetic and pharmacodynamic technologies in hypothesis-testing clinical trials of innovative therapies. J. Natl. Cancer Inst. 98, 580–598 (2006)
Attard, G. et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 69, 2912–2918 (2009)
de Bono, J. S. et al. Potential applications for circulating tumor cells expressing the insulin-like growth factor-I receptor. Clin. Cancer Res. 13, 3611–3616 (2007)
Grantab, R., Sivananthan, S. & Tannock, I. F. The penetration of anticancer drugs through tumor tissue as a function of cellular adhesion and packing density of tumor cells. Cancer Res. 66, 1033–1039 (2006)
Propper, D. J. et al. Use of positron emission tomography in pharmacokinetic studies to investigate therapeutic advantage in a phase I study of 120-hour intravenous infusion XR5000. J. Clin. Oncol. 21, 203–210 (2003)
Minchinton, A. I. & Tannock, I. F. Drug penetration in solid tumours. Nature Rev. Cancer 6, 583–592 (2006)
Mandrekar, S. J. & Sargent, D. J. Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges. J. Clin. Oncol. 27, 4027–4034 (2009)
Taube, S. E. et al. A perspective on challenges and issues in biomarker development and drug and biomarker codevelopment. J. Natl. Cancer Inst. 101, 1453–1463 (2009)
Fong, P. C. et al. Inhibition of Poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009)Clinical evidence of the efficacy of a synthetic lethal therapeutic approach for cancer.
Druker, B. J. et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344, 1031–1037 (2001)Landmark paper demonstrating efficacy of imatinib in CML.
Soda, M. et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature 448, 561–566 (2007)
van Oosterom, A. T. et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 358, 1421–1423 (2001)
Verweij, J. et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364, 1127–1134 (2004)
Von Hoff, D. D. et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N. Engl. J. Med. 361, 1164–1172 (2009)
Jordan, V. C. Is tamoxifen the Rosetta stone for breast cancer? J. Natl. Cancer Inst. 95, 338–340 (2003)
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001)
Karapetis, C. S. et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 359, 1757–1765 (2008)
Mok, T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009)
Kantarjian, H. et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N. Engl. J. Med. 354, 2542–2551 (2006)
Shah, N. P. et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 305, 399–401 (2004)
Talpaz, M. et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 354, 2531–2541 (2006)
Sarker, D., Reid, A. H. M., Yap, T. A. & de Bono, J. S. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin. Cancer Res. 15, 4799–4805 (2009)
Yap, T. A. et al. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr. Opin. Pharmacol. 8, 393–412 (2008)
Ingle, J. N. et al. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N. Engl. J. Med. 304, 16–21 (1981)
Attard, G., Ang, J. E., Olmos, D. & de Bono, J. S. Dissecting prostate carcinogenesis through ETS gene rearrangement studies: implications for anticancer drug development. J. Clin. Pathol. 61, 891–896 (2008)
Reid, A. H. M. et al. Molecular characterisation of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer. Br. J. Cancer 102, 678–684 (2010)
Attard, G., Cooper, C. S. & de Bono, J. S. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer Cell 16, 458–462 (2009)
Attard, G. et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 26, 4563–4571 (2008)First clinical evidence to definitely prove that advanced castration resistant prostate cancer remains hormone driven.
Attard, G. et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 27, 3742–3748 (2009)
Tomlins, S. A. et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature 448, 595–599 (2007)
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005)
Attard, G. & de Bono, J. S. Prostate cancer: PSA as an intermediate end point in clinical trials. Nature Rev. Urol. 6, 473–475 (2009)
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009)
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 92, 205–216 (2000)
Budd, G. T. et al. Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin. Cancer Res. 12, 6403–6409 (2006)
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004)
de Bono, J. S. et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 14, 6302–6309 (2008)
De Giorgi, U. et al. Circulating tumor cells and [18F]fluorodeoxyglucose positron emission tomography/computed tomography for outcome prediction in metastatic breast cancer. J. Clin. Oncol. 27, 3303–3311 (2009)
Olmos, D. et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Ann. Oncol. 20, 27–33 (2009)
Dowsett, M. et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J. Natl. Cancer Inst. 99, 167–170 (2007)
Greystoke, A. et al. Optimisation of circulating biomarkers of cell death for routine clinical use. Ann. Oncol. 19, 990–995 (2008)
Rosen, J. M. & Jordan, C. T. The increasing complexity of the cancer stem cell paradigm. Science 324, 1670–1673 (2009)
Buyse, M. et al. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J. Clin. Oncol. 25, 5218–5224 (2007)
Buyse, M., Molenberghs, G., Burzykowski, T., Renard, D. & Geys, H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics 1, 49–67 (2000)
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005)Preclinical evidence indicating the potential of synthetic lethality as a therapeutic strategy for BRCA-mutation associated cancers.
Reid, A. H. M. et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J. Clin. Oncol. 28, 1489–1495 (2010)
Flaherty, K. T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010)
Bollag, G. et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature advance online publication doi:10.1038/nature09454 (7 September 2010)
Kwak, E. L. et al. Clinical activity observed in a Phase I dose escalation trial of an oral c-met and ALK inhibitor, PF-02341066. J. Clin. Oncol. (Meeting abstracts) 27, 3509 (2009)
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the generation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
de Bono, J., Ashworth, A. Translating cancer research into targeted therapeutics. Nature 467, 543–549 (2010). https://doi.org/10.1038/nature09339
Issue Date:
DOI: https://doi.org/10.1038/nature09339
This article is cited by
-
Patient Preferences in Targeted Pharmacotherapy for Cancers: A Systematic Review of Discrete Choice Experiments
PharmacoEconomics (2023)
-
Clinical prospects of WRN inhibition as a treatment for MSI tumours
npj Precision Oncology (2022)
-
Severe testing with high-dimensional omics data for enhancing biomedical scientific discovery
npj Systems Biology and Applications (2022)
-
Accuracy and reproducibility of somatic point mutation calling in clinical-type targeted sequencing data
BMC Medical Genomics (2020)
-
Tumor microenvironment-targeted nanoparticles loaded with bortezomib and ROCK inhibitor improve efficacy in multiple myeloma
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.