Abstract
Although circumcision reduces male acquisition of human immunodeficiency virus type-1 (HIV-1) by 60%, the initial mechanisms of HIV-1 transmission at the foreskin remain elusive. We have established two novel and complementary models of the human adult foreskin epithelium, namely, ex vivo foreskin explants and in vitro reconstructed immunocompetent foreskins. In these models, efficient HIV-1 transmission occurs after 1 h of polarized exposure of the inner, but not outer, foreskin to mononuclear cells highly infected with HIV-1, but not to cell-free virus. HIV-1-infected cells form viral synapses with apical foreskin keratinocytes, leading to polarized budding of HIV-1, which is rapidly internalized by Langerhans cells (LCs) in the inner foreskin. In turn, LCs migrate toward the epidermis–dermis interface to form conjugates with T cells, thereby transferring HIV-1. Seminal plasma mixed with cervicovaginal secretions inhibits HIV-1 translocation. This set of results rationalizes at the cellular level the apparent protective outcome of circumcision against HIV-1 acquisition by men.
Similar content being viewed by others
Introduction
The main entry portals of human immunodeficiency virus type-1 (HIV-1) during its sexual transmission are the mucosal surfaces of the female and male genital tracts, which are covered by different types of epithelial barriers. Simple epithelia consist of monolayers of epithelial cells having a polarized plasma membrane, separated by tight junctions into two domains, the apical and the basal, with different protein and lipid composition. In contrast, stratified epithelia are made of multiple layers of nonpolarized epithelial cells in which various immune cells are inserted.1
The first steps of mucosal HIV-1 transmission have been investigated, to date, primarily in the different epithelia present in the female genitals and the gastrointestinal tract. In simple epithelia, such as the endocervix, rectum, and intestine, we and others have previously shown that HIV-1 may cross in vitro such an epithelial barrier by the transcellular pathway of transcytosis.2, 3, 4, 5, 6 This process involves the formation, at the apical pole, of a viral synapse between HIV-1-infected cells and epithelial cells, which induces polarized virus budding at the contact area between the two cell types.2, 5, 7, 8, 9 The newly budded virus is then rapidly internalized by the epithelial cells (without infecting the epithelial cells, as is the case in vivo), transcytosed toward the basal pole, and released, still infectious, into the basal environment.2 Importantly, early studies including our own have shown that HIV-1 entry into mucosal epithelial cells is much more efficient when HIV-1 particles bud locally after contact between HIV-1-infected cells and uninfected epithelial cells, compared with cell-free HIV-1.2, 5, 10
In stratified epithelia, such as the vagina, exocervix, and anus, early HIV-1 transmission is considered to involve epidermal Langerhans cells (LCs). These professional antigen-presenting cells (APCs), integrated within these epithelia, sample the mucosal surface for incoming foreign pathogens. In turn, LCs migrate to secondary lymphoid organs to present processed antigens to T cells (for review see Merad et al.11). Owing to their close proximity to the mucosal surface in normal tissue, LCs have been suggested to be among the first cells to capture and/or become infected with HIV-1 during its mucosal transmission, before transferring HIV-1 to T cells in the proximal lymph nodes (reviewed in Hladik and McElrath12 and de Witte et al.13). HIV-1 enters purified LCs in vitro using its envelope glycoprotein subunit gp120 that binds the LC-specific C-type lectin langerin,14, 15, 16 inducing in turn either virus capture and infection of LCs for further transfer to T cells or virus internalization and degradation depending on the viral load.16
Surprisingly, how HIV-1 enters the male genitals remains elusive. At the population level, more than 40 epidemiological studies,17 confirmed by three recent randomized controlled trials,18, 19, 20 have concluded that circumcision offers a 60% reduction in HIV-1 transmission from infected women to men. However, a precise description of the initial events of HIV-1 transmission in the different epithelia of the male genital tract is currently missing.
In the adult human foreskin, the apical surface of the outer foreskin is more keratinized than the inner one.21, 22, 23 Such a higher degree of foreskin keratinization has been suggested to provide a protective barrier against HIV-1 transmission.22, 24 Potential HIV-1 target cells expressing CD4, the principal receptor for HIV-1, and the coreceptor CCR5 are present in the inner and outer foreskin.21, 22, 23, 25, 26, 27 To date, only two published studies of foreskin infection with HIV-1 have evaluated the entry of high doses of cell-free HIV-1 inocula at time points of >24 h. First, on polarized infection of agarose-sealed foreskin tissue explants, the inner foreskin appears susceptible to infection with R5, but not X4, cell-free HIV-1.22 However, sealing efficiency and thereby polarization of the infection in this system, has been previously questioned.28 Second, and more recently, using nonpolarized foreskin tissue explants in which HIV-1 can access to both tissue surfaces, inner and outer foreskins were infected to a similar degree with R5, but not X4, cell-free HIV-1.27
Sexual transmission of HIV-1 is mediated in vivo by genital fluids, such as semen and cervicovaginal secretions (CVSs), which contain both cell-free HIV-1 and HIV-1-infected cells. However, the infectious potential of HIV-1-infected cells has been little evaluated in pluristratified mucosa.29, 30, 31 Furthermore, the role of genital fluids themselves in HIV-1 transmission remains unclear. Recent studies showed either an inhibitory32 or an enhancing33 effect of seminal plasma (SP) on HIV-1 transmission. To date, no studies have addressed the possible contribution of genital fluids to HIV-1 transmission in the male genital tract.
A principal impediment in studying mucosal HIV-1 transmission is the lack of proper in vitro viral transmission model systems that reflect the complex in vivo situation in the humans, as nonprimate animals (models/tissues) are not susceptible to HIV-1.
To describe the initial events of HIV-1 entry at the foreskin, we therefore developed two novel models of the foreskin epithelium, namely, ex vivo polarized inner and outer foreskin explants and immunocompetent pluristratified foreskin epithelia reconstructed in vitro from isolated primary inner or outer foreskin cells. These models were inoculated comparatively in a polarized manner with either cell-free HIV-1 or HIV-1-infected cells for 1 h. In addition, the role of male and female genital fluids in HIV-1 entry was evaluated. Our studies allowed for the in vitro investigation of the early events taking place during the first hour after exposure of the foreskin epithelium to HIV-1, in conditions mimicking the in vivo viral transmission during sexual intercourse.
Results
Quantification of cells involved in HIV-1 transmission in the human foreskin
In addition to LCs, T cells, dendritic cells (DCs), and macrophages are potential targets for HIV-1. To investigate the presence, distribution, and density of these various cells in the foreskin epithelium, sections derived from either inner or outer normal foreskin tissues were stained for cell-specific markers, namely, langerin for LCs, CD3 for T cells, DC-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) for DCs, and CD68 for macrophages.
In inner foreskin, langerin+ cells were present almost exclusively in the epidermis (Figure 1a). The majority of CD3+ cells were confined to the dermis (Figure 1b). In contrast, DC-SIGN+ and CD68+ cells were detected throughout the dermis but not in the epidermis (Figure 1c and d).
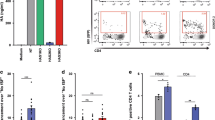
Identification and quantification of potential human immunodeficiency virus type-1 (HIV-1) target cells in normal human foreskin. Representative images of normal inner foreskin tissues processed for immunohistochemistry using antibodies (Abs) to langerin (a), CD3 (b), dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) (c), and CD68 (d). Cells were visualized with either 3-amino-9-ethylcarbazole (AEC red; a and c) or di-amino benzidine (DAB brown; b and d) peroxidase substrates. Inserts show control negative staining with matched isotype controls. Bars=10 μm. (e) Cell densities per mm2 of either epidermis for langerin+ and CD3+ cells or dermis for CD3+, DC-SIGN+, and CD68+ cells; means±s.e.m. derived of n=10–15 normal foreskin tissues. For each foreskin, staining was performed in parallel in inner and outer parts and cells were counted in a minimum of 10 fields.
When compared with outer foreskin, the density of epidermal LCs (that is, langerin+ cells per mm2 epidermis), as well as the density of epidermal and dermal T cells (that is, CD3+ cells per mm2 epidermis or dermis) in inner foreskin was significantly higher (Figure 1e). In contrast, no significant difference was observed in the density of dermal DC-SIGN+ DCs and CD68+ macrophages between inner and outer foreskins (Figure 1e).
In addition, expression of the HIV-1 coreceptor CCR5 on both epidermal LCs and T cells was evaluated by flow cytometry. Hence, single-cell suspensions prepared from normal foreskin epidermal sheets were double stained with anti-CCR5-fluorescein isothiocyanate (FITC) and either anti-CD3 allophycocyanin (APC) or anti-langerin-APC monoclonal antibodies (mAbs). The percentages of CCR5-expressing LCs (out of the total langerin+ cells) or T cells (out of the total CD3+ cells) were then evaluated. In inner foreskin, CCR5 was expressed on 20.9±1.6% LCs and 35.5±11.4% T cells. In outer foreskin, only 6.1±3.1% LCs and 5.3±3.6% T cells were CCR5 positive (P=0.0066 and 0.0325, respectively, n=3).
As LCs and T cells have been proposed to participate in mucosal HIV-1 foreskin transmission,22 these results suggest that the inner foreskin may be more susceptible to HIV-1 infection compared with the outer foreskin.
Development of a novel ex vivo explant model of the foreskin epithelium
During the sexual transmission of HIV-1, virus entry takes place selectively through the apical/mucosal surface of the epithelium. Thus, to mimic the early events in HIV-1 transmission, any mucosal tissue explant model must be designed to permit restricted access of the virus to the apical side. Accordingly, in our model, pieces of normal adult human foreskin tissues from either inner or outer foreskin were placed with their epidermal/apical side facing up on top of a permeable membrane in a two-chamber system. To restrict HIV-1 exposure through the apical side, hollow plastic cloning ring cylinders were adhered tightly to the epidermal surface of each tissue explant, using surgical glue (Figure 2a), resulting in the creation of a sealed apical chamber that permits for subsequent polarized inoculation of HIV-1. Sealing efficiency was routinely monitored as described in the Methods.
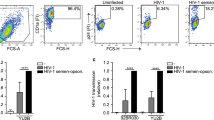
(a) A schematic representation of the experimental explant system developed to investigate short-term and polarized human immunodeficiency virus type-1 (HIV-1) infection of the human foreskin. Round 8-mm pieces of foreskin tissue explants are placed on top of a porous polycarbonate (PC) filter in a two-chamber system, and a hollow plastic cylinder is adhered to the apical surface of each explant with the aid of surgical glue. HIV-1 is later added in a polarized manner to the inner space of the cylinder. (b) HIV-1-infected peripheral blood mononuclear cells (PBMCs) form viral synapses with apical keratinocytes in foreskin explants. Electron micrographs of inner foreskin explant exposed for 1 h to HIV-1-infected PBMCs. Shown is the same HIV-1-infected PBMC in two serial sections, forming a close contact with the apical keratinocytes. Bars=1 μm. (c) HIV-1 entry into inner, but not outer, foreskin explants. Three-dimensional reconstructions from confocal image stacks of inner (left) and outer (right) foreskin explants, after 1 h exposure to either noninfected PBMCs (white arrows in top panel) or HIV-1 V29-infected PBMCs (yellow arrows in middle panel). Horizontal and vertical lines in middle panel represent the localization of the sectioned layers that are shown below and to the right of each image. Bottom panel shows higher magnifications of the epidermis with the xyz planes rotated. Sections were stained with a mixture of several anti-HIV-1 monoclonal antibodies (mAbs), followed by fluorescein isothiocyanate-conjugated anti-human- and mouse-IgG Ab. Single HIV-1 virions are detected as green dots (yellow arrowheads). Cell nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole). Bars=5 μm. Images are representative of three independent experiments. (d) Means±s.e.m. number of HIV-1 virions stained with 2F5 mAb in three independent inner (light gray bar) and outer (dark gray bar) foreskin explants following 1 h exposure to HIV-1 V29-infected PBMCs.
HIV-1-infected cells form viral synapses with apical keratinocytes in foreskin explants
Previous studies, including our own, have shown that HIV-1-infected cells form direct contacts with epithelial cells at the apical surface of mucosal tissues, resulting in the creation of viral synapses. This leads to the polarized budding of newly formed viral particles, most likely increasing the local virus concentration at the contact area between the cells and thus virus infectivity.2, 5, 10
At the ultrastructural level, when foreskin explants were exposed for 1 h to peripheral blood mononuclear cells (PBMCs) infected with a primary R5 clade B HIV-1 isolate (termed herein as V29), contacts were identified between HIV-1 V29-infected PBMCs and the apical surface of inner foreskin explants (Figure 2b). Such contacts were not observed when foreskin explants were exposed to noninfected PBMCs serving as control.
In addition, at the light microscopy level, a higher density of contacts was observed after 1 h exposure to HIV-1 V29-infected PBMCs, compared with similar exposure to noninfected PBMCs. Thus, in inner foreskin, the average density of contacts per cm of apical length was 226±59 compared with 108±83 for HIV-1 V29-infected PBMCs and noninfected PBMCs, respectively (n=3; P=0.0410). Similarly, in outer foreskin, the densities were 839±210 compared with 78±39 for HIV-1 V29-infected PBMCs and noninfected PBMCs, respectively (n=3; P=0.0118). These results are in line with previous studies showing that HIV-1-infected cells are more adhesive on intestinal and cervical epithelial cells compared with noninfected cells.8 The interaction between HIV-1 V29-infected PBMCs and apical foreskin keratinocytes therefore represents genuine viral synapses,34 similar to the ones previously reported to be formed between HIV-1-infected cells and mucosal epithelia (as reviewed in Phillips10 and shown more recently in Alfsen et al.5), which are induced after exposure of apical foreskin keratinocytes to HIV-1-infected cells.
HIV-1 enters inner foreskin explants, but is trapped within the thick layer of keratin of outer foreskin explants
To detect HIV-1 entry into the foreskin, inner and outer foreskin explants were exposed for 1 h to either HIV-1 V29-infected PBMCs or noninfected PBMCs as control. Semi-thick 4 μm sections derived from these explants were then stained for HIV-1 with either the mAb 2F5 that recognizes HIV-1 gp41, a cocktail of several additional mAbs directed against HIV-1 gp41/gp120/gp160/p24, or human sera derived from HIV-1-infected individuals, and examined by fluorescent and confocal microscopy.
In inner foreskin explants, numerous HIV-1 particles were detected within the epidermis by a cocktail of anti-HIV-1 mAbs after exposure to HIV-1 V29-infected PBMCs (Figure 2c, middle and bottom left). In contrast, in outer foreskin explants, fewer HIV-1 particles entered the epidermis, and most virions were confined to the thick layer of keratin covering the outer foreskin surface (Figure 2c, middle and bottom right). No staining was observed in foreskin explants (either inner or outer) exposed to noninfected PBMCs (Figure 2c, top). Similar results were obtained when the foreskin explants were stained with the 2F5 mAb or HIV-1+ human sera (data not shown). Quantifying the number of 2F5-stained virions per mm2 of epidermis showed that 563±150 compared with 197±135 virions entered the epidermis of inner and outer foreskins, respectively (Figure 2d). These results suggest that the higher degree of keratinization in the outer foreskin provides a physical barrier to HIV-1 entry into the foreskin.
Short-term polarized exposure of inner foreskin explants to HIV-1 induces changes in the density and distribution of LCs
To evaluate the role of LCs in the first hour of HIV-1 transmission in the foreskin, inner and outer foreskin explants where inoculated in a polarized manner with either cell-free HIV-1 V29 (compared with RPMI medium) or with one million HIV-1 V29-infected PBMCs, a quantity similar to that found in genital fluids of HIV-1-infected individuals35 (compared with noninfected PBMCs). Importantly, viral load in genital fluids of HIV-1-infected individuals is known to vary significantly with time in a single individual, even within the same day. Thus, to mimic such variation, two different loads of virus were applied comparatively to the foreskin explants (see the Methods).
After 1 h of polarized exposure of such foreskin explants, semi-thick sections were immunolabeled with an anti-langerin antibody (Ab) and fluorescence microscopy was used to detect epidermal LCs. In parallel explants, epidermis was separated from the dermis, single-cell suspensions were prepared from triplicate epidermal sheets, and the cells were labeled with anti-langerin Ab and analyzed by flow cytometry. Of note, these two methodological approaches provide complementary information: langerin staining in sections reveals the spatial localization and density of LCs in a given part of the epidermis, whereas langerin staining in epidermal single-cell suspensions reveals the percentage of LCs among the total cells within the whole epidermis.
When PBMCs weakly infected with HIV-1 V29 were applied for 1 h, the density of epidermal LCs, namely, langerin+ cells per mm2 epidermis, in both inner and outer foreskin explants remained similar to that observed in normal tissue (compare Figure 3a with Figure 1e). In contrast, LC density decreased by a third on inoculation with noninfected PBMCs, in inner, but not outer, foreskin explants (Figure 3a). Similar results were obtained when LC numbers were evaluated by flow cytometry of epidermal single-cell suspensions (Figure 3b) or staining with anti-CD1a Ab (data not shown). Exposure to a low concentration of cell-free HIV-1 V29 had the same effect in inner, but not outer, epidermal single-cell suspensions (Supplementary Figure 1a). These results suggest that in inner foreskin, low concentration of HIV-1 leads to retention of LCs within the epidermis during the first hour of viral exposure, regardless of the source of the virus. In contrast, the decrease in LC density after exposure to noninfected PBMCs probably represents the natural emigration of LCs from the epithelium, a known feature of these migratory cells that is routinely used experimentally for their isolation from mucosal epithelia.
(a, b) Retention of Langerhans cells (LCs) in the epidermis following exposure to low concentration of human immunodeficiency virus type-1 (HIV-1). (a) Representative fluorescent microscopy images of inner (top panel) and outer (bottom panel) foreskin explants, exposed for 1 h to either peripheral blood mononuclear cells (PBMCs) weakly infected with HIV-1 (left) or noninfected PBMCs (right). Explants were stained with goat-anti-human langerin antibody (Ab), followed by fluorescein isothiocyanate (FITC)-conjugated anti-goat-IgG Ab. Green arrowheads point to langerin+ cells. Cell nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole). Bars=10 μm. Images are representative of three independent experiments. The calculated means±s.e.m. density of epidermal LCs (that is, langerin+ cells per mm2 epidermis) derived of three independent experiments are also shown (graph). (b) Representative flow cytometry histograms of cells in epidermal single-cell suspensions prepared from inner foreskin explants exposed for 1 h to PBMCs weakly infected with HIV-1 (top left) or noninfected PBMCs (bottom left). Cells were stained with either phycoerythrin-conjugated anti-human langerin mAb (thick lines) or matched isotype control (thin lines). Numbers represent the percentage of positive cells in the M1 region. Images are representative of three independent experiments. The means±s.e.m. fold decreases in the percentage of epidermal langerin+ cells (calculated as (percentage of langerin+ cells after exposure to noninfected PBMCs/percentage of langerin+ cells after exposure to PBMCs weakly infected with HIV-1 V29)), in inner (light gray bar) and outer (dark gray bar) foreskin explants, are derived of three experiments (graph). (c, d) Calculated mean folds±s.e.m. of either epidermal LCs density in foreskin explants (c) or percentage in epidermal single-cell suspensions (d), after 1 h exposure of inner (light gray bars) and outer (dark gray bars) foreskin explants to noninfected PBMCs (compared with PBMCs highly infected with HIV-1 V29) derived of three experiments. (e) Representative fluorescent microscopy images of inner foreskin explants, exposed for 1 h to either PBMCs highly infected with HIV-1 (left) or noninfected PBMCs (right). Explants were stained with goat-anti-human langerin Ab, followed by FITC-conjugated anti-goat-IgG Ab. Cell nuclei were counterstained with DAPI. Bars=20 μm. Images are representative of three independent experiments. Distances between each individual langerin-positive cell and the mucosal surface (orange dotted line) were measured, and the calculated means±s.e.m. distance of epidermal LCs from the mucosal surface derived of three independent experiments are shown (graph).
Conversely, no changes in either the density of epidermal LCs (Figure 3c) or the percentage of LCs in epidermal single-cell suspensions (Figure 3d) occurred in both inner and outer foreskin explants, following exposure to PBMCs highly infected with HIV-1 V29 or high concentration of cell-free HIV-1 V29 (Supplementary Figure 1b).
However, 1 h exposure of inner, but not outer, foreskin explants to PBMCs highly infected with HIV-1 V29 modified the spatial distribution of LCs within the epidermis (Figure 3e). Hence, the mean distance of LCs from the apical surface in inner foreskin shortened significantly after exposure to PBMCs highly infected with HIV-1 V29 compared with exposure to noninfected PBMCs (19.1±3.9 μm compared with 26.3±3.8 μm, respectively; calculated from three independent experiments). In outer foreskin, no such change was observed, with the respective mean distances being 23.3±7.5 μm and 20.3±3.6 μm after exposure to HIV-1-infected or noninfected PBMCs, respectively.
Finally, in the dermis, the percentage of single CD3+ T cells and DC-SIGN+ DCs did not change after short-term exposure to either cell-free HIV-1 V29 or HIV-1 V29-infected cells in any of these explants (data not shown), as compared with controls.
Taken together, these results suggest that LCs are the initial target cells interacting with HIV-1 in the inner foreskin. Moreover, depending on the nature and concentration of the HIV-1 inocula, LCs are either retained in the mucosal side of the epidermis or migrate toward the apical surface.
LC–T cell conjugates permit transfer of HIV-1 from LCs to T cells
Previous studies showed that not only could single LCs and T cells emigrate from skin explants but so could LC–T cell conjugates, in which active transfer of HIV-1 from LCs to T cells can take place.36, 37, 38, 39, 40 We therefore investigated whether such LC–T cell conjugates formed within the foreskin, by double labeling epidermal single-cell suspensions for langerin and CD3. Exposure of inner, but not outer, foreskin to PBMCs highly infected with HIV-1 V29 for 1 h resulted in an increase in the percentage of LC–T cell conjugates compared with exposure to noninfected PBMCs (Figure 4a). In contrast, LC–T cell conjugate formation was not detected after similar exposure to a high concentration of cell-free HIV-1 V29 of either part of the foreskin (Supplementary Figure 1c).
Langerhans cells (LCs) transfer human immunodeficiency virus type-1 (HIV-1) to T cells across conjugates. (a) Higher percentage of epidermal LC–T cell conjugates following exposure to peripheral blood mononuclear cells (PBMCs) highly infected with HIV-1 V29. Representative flow cytometry profiles of epidermal single-cell suspensions double stained with CD3-allophycocyanin (APC) and langerin-phycoerythrin (PE) monoclonal antibodies (mAbs). Cells were first gated on CD3+ cells (R1 gate, top left profile, insert shows staining with matched isotype control). The percentages of cells in R1 that show high forward scatter and are also langerin+ (compared with matched isotype control, top right profile) were evaluated in suspensions prepared from inner foreskin explants exposed for 1 h to PBMCs highly infected with HIV-1 V29 (bottom right) or noninfected PBMCs (bottom left). Numbers represent the percentage of CD3+/langerin+/high FSC conjugates. Images are representative of three independent experiments. The calculated mean±s.e.m. fold increases in the percentage of epidermal LC–T cell conjugates after 1 h exposure of inner (light gray bar) and outer (dark gray bar) foreskin explants to PBMCs highly infected with HIV-1 V29 (normalized against noninfected PBMCs) derived from three independent experiments are also shown (graph). (b–f) Electron microscopy images of inner foreskin explant exposed for 1 h to HIV-1 infected PBMCs. (b) LC–T cell conjugates are detected within the epidermis at the epidermal–dermal interface above the basement membrane (black dotted line); Bar=1 μm. (c) Higher magnification of the single starred frame in (b), showing a short rod-shaped Birbeck granule in the LC cytoplasm (black arrow), as well as an HIV-1 particle within a vesicular compartment (white arrowhead); Bar=0.2 μm. (d, e) Higher magnifications of the double and triple starred frames in (b), showing HIV-1 particles (white arrowheads, higher magnification insert in (d)) in the contact area between the LC and T cell; Bar=0.1 μm. (f) Electron microscopy image of inner foreskin explant exposed for 1 h to noninfected PBMCs, showing an isolated and nonconjugated T cell within the epidermis at the epidermal–dermal interface above the basement membrane (black dotted line); Bar=1 μm.
Next, the localization of LCs and T cells within the inner foreskin was investigated at the ultrastructural level. When inner foreskin explants were exposed for 1 h to PBMCs highly infected with HIV-1, conjugates between LCs, identified by their typical Birbeck granules in the cytoplasm, and T cells, identified by the typical morphology and shape/size of their nucleus, were detected in the epidermis, positioned above the basement membrane at the epidermal–dermal interface (Figure 4b). HIV-1 virions were detected either in distinct vesicular compartments in the LC cytoplasm (Figure 4c) or at the contact area between the two cell types (Figure 4d and e). This suggests that the virus is first internalized by LCs, and then transferred to T cells across a viral synapse. In contrast, only isolated T cells were detected at the epidermal–dermal interface on exposure of inner foreskin explants to noninfected PBMCs (Figure 4f).
Development of a novel in vitro immunocompetent reconstructed model of the foreskin epithelium
A major technical limitation in studying HIV-1 entry at mucosal sites using tissue explants is the short time frame during which the tissue retains structural and morphological integrity. Moreover, owing to tissue sectioning, migratory cells, such as LCs and T cells, quickly emigrate out of these explants, thus limiting the evaluation of their exact role in early HIV-1 transmission. Therefore, we sought to establish an alternative in vitro model to study the mechanisms of early HIV-1 transmission across the foreskin.
Establishment of such reconstructed foreskin models (see the Methods) is based on our previous successful reconstruction of an immunocompetent vaginal epithelium29 and on methodologies used thus far for the development of skin equivalents (reviewed in MacNeil41). Briefly, as schematized in Figure 5a, establishment of collagen-rich dermis-like structures is initiated by seeding purified primary inner or outer foreskin fibroblasts in the apical compartment of a two-chamber system, followed by culture under submerged conditions in the presence of growth factors and supplements that were previously described to promote collagen production.42 As a result, after 21 days, multilayered fibroblasts expand on both sides of the filter. Purified primary human inner or outer foreskin keratinocytes are then seeded on the dermis-like sheets of fibroblasts, together with immature LCs/DCs that are differentiated in vitro from CD14+ blood monocytes. Of note, owing to technical limitations, fibroblasts and keratinocytes used for a given reconstruction are routinely derived from foreskins of different individuals, thus preventing the incorporation of T cells into these reconstructions. As keratinocytes differentiate upon culture at the air–liquid interface43, 44 and to mimic the structure of either the low keratinized inner or highly keratinized outer foreskin epidermis,21, 22, 23 foreskin reconstructions are cultured for various time periods at the air–liquid interface.
In vitro reconstruction of the human foreskin epithelium. (a) A schematic representation of the experimental protocol used to reconstruct in vitro an immunocompetent foreskin epithelium, consisting of primary human foreskin fibroblasts and keratinocytes, as well as immature Langerhans cells (LCs)/dendritic cells (DCs) (D; day of culture). (b) Masson’s trichrome staining of normal and reconstructed inner (left) and outer (right) foreskins. The white double-headed arrows emphasize the increased cell number/thickness of the keratinocytes layers in outer compared with inner foreskin reconstructions. Fb, fibroblasts; Ker, keratinocytes. (c) Fluorescence microscopy images of normal and reconstructed inner (left) and outer (right) foreskins, stained with mouse-anti-human CK14 monoclonal antibody (mAb), followed by fluorescein isothiocyanate-conjugated anti-mouse antibody (Ab). Cell nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole). (d) Immunohistochemistry of normal and reconstructed inner (left) and outer (right) foreskins, stained for the expression of the LC/DC common marker CD1a (brown; DAB peroxidase substrate). Black arrows point to CD1a+ cells in foreskin reconstructions. All images are representative of at least three experiments performed with different normal or reconstructed foreskins for each morphological/structural characteristic. Bars=10 μm.
When compared with normal foreskins, reconstructions present similar morphological and structural characteristics (Figure 5b–d). Dermis-like structures rich in collagen fibers are detected by Masson's trichrome staining in both inner and outer foreskin reconstructions (Figure 5b). Reconstructions of outer foreskin show more layers of differentiated keratinocytes and a thicker epidermis compared with inner foreskin reconstructions (Figure 5b). These features are also observed at the ultrastructural level by electron microscopy (Supplementary Figure 2a). Specific immunolabeling of semi-thick sections shows that expression of the epithelial differentiation cell markers cytokeratin (CK) 14 (Figure 5c), as well as CK10 and involucrin (Supplementary Figure 2b, c) in reconstructed foreskin resembles that of normal foreskin. Finally, CD1a+ LCs/DCs, detected by immunohistochemistry, are integrated into the reconstructed foreskin (Figure 5d). Evaluating the density of CD1a+ cells (that is, CD1a+ cells per mm2 epidermis) shows that 1441±62 and 914±83 cells per mm2 are integrated in inner and outer foreskin reconstructions, respectively (n=3; P=0.0183). These values are in agreement with that calculated for normal epidermis: 1370±187 and 677±120 cells per mm2 in normal inner and outer foreskins, respectively (n=3; P=0.0194).
Taken together, the above findings show that we successfully established novel immunocompetent in vitro models of both inner and outer foreskins, with the structural and morphological characteristics of normal foreskin. Foreskin reconstructions were next inoculated with high doses of cell-free HIV-1 or HIV-1-highly infected cells and the effects of these two types of viral inoculation were compared.
HIV-1 buds locally at viral synapses formed in foreskin reconstructions
When inner and outer foreskin reconstructions were exposed for 1 h to HIV-1 V29-infected PBMCs, a higher number of infected cells adhered to the apical surface compared with noninfected PBMCs. Thus, in inner foreskin, the average density of viral synapses per cm of apical length was 158±60 compared with 54±54 for HIV-1-infected PBMCs and noninfected PBMCs, respectively (n=3; P=0.0088). Similarly, in outer foreskin, the densities were 245±98 compared with 54±37 for HIV-1 V29-infected PBMCs and noninfected PBMCs, respectively (n=3; P=0.0466). These results are in line with our observations made with foreskin explants (Figure 2). At the ultrastructural level, viral synapses were identified at the apical surface of foreskin reconstructions exposed to HIV-1 V29-infected PBMCs (Figures 6a and c), as already shown for foreskin explants (Figure 2b). HIV-1 virions were detected at the contact area between the virus-infected cells and foreskin keratinocytes (Figure 6b). These virions had a typical morphology of immature doughnut-shaped particles with electron-lucent centre, as previously described.5, 45, 46, 47
(a–c) Electron microscopy images of outer foreskin reconstruction exposed for 1 h to human immunodeficiency virus type-1 (HIV-1)-infected peripheral blood mononuclear cells (PBMCs). Shown are two different HIV-1-infected PBMCs in contact with the apical keratinocytes (a, c). Higher magnification of the single starred frame in a is presented in b and shows HIV-1 particles (white arrowheads, higher magnification inserts) in the contact area between the HIV-1-infected PBMC and apical keratinocyte. (d–g) HIV-1 translocation across foreskin reconstructions. (d, e) Outer and inner foreskin reconstructions were exposed for 1 h to either HIV-1 V29-infected PBMCs (d) or cell-free HIV-1 V29 (e). Apical and basal supernatants were then collected, and the amount of HIV-1 was measured by p24 enzyme-linked immunosorbent assay (ELISA). Results are derived from 3 to 5 different paired reconstructions of either outer/inner foreskins, and are presented for each experiment as the percentage of HIV-1 translocation (p24 in basal chamber/p24 in apical chamber × 100). P=0.0314 and 0.0500, inner vs. outer, for cell-associated and cell-free HIV-1, respectively. (f, g) Outer and inner foreskin reconstructions were exposed for 1 h to decreasing amounts (that is, 1, 0.25, 0.1, and 0.025 × 106) of HIV-1 V29-infected PBMCs (f) or cell-free HIV-1 V29 (g), and the percentage of HIV-1 translocation was evaluated as described above. Shown are means±s.d. derived from two independent experiments performed. *P<0.0001 and **P<0.0001, inner vs. outer. (h) A mixture of seminal plasma (SP) and cervicovaginal secretions (CVS) decreases HIV-1 translocation. Inner foreskin reconstructions were exposed for 1 h to HIV-1 V29-infected PBMCs, which were resuspended either in RPMI medium alone or in RPMI medium in the presence of 1:10 diluted SP and/or 1:4 diluted CVS. Apical and basal supernatants were then collected, the amount of HIV-1 was measured by p24 ELISA, and the percentage of HIV-1 translocation (p24 in basal chamber/p24 in apical chamber × 100) was calculated separately for each sample. Results are presented as the normalized percentage of translocation (percentage of translocation in the presence of genital fluids/percentage of translocation with RPMI medium alone), and are derived from seven independent inner foreskin reconstructions.
Exposure of inner foreskin reconstructions to HIV-1-infected cells results in efficient translocation of HIV-1
In all secretions vectorizing HIV-1, the virus is present either as cell-free particles or as virus-infected cells. To compare the relative infectious potential of these different sources of HIV-1, inner and outer foreskin reconstructions were exposed either to HIV-1 V29-infected PBMCs or to cell-free HIV-1 V29. Noninfected PBMCs or RPMI medium alone served as controls, respectively. After 1 h exposure, translocation of HIV-1 to the basal compartment of the chambers was evaluated by a p24 enzyme-linked immunosorbent assay (ELISA) and the mean±s.e.m. percentage of translocation was calculated. In inner foreskin, translocation of HIV-1 induced by contact with HIV-1-infected cells (Figure 6d) was 90.5 times more efficient compared with translocation of cell-free HIV-1 (Figure 6e). Thus, 8.15±3.12% of HIV-1 translocated when HIV-1 was produced apically by infected cells compared with 0.09±0.04% for cell-free HIV-1 (P=0.0483). Similarly, in outer foreskin, translocation induced by HIV-1-infected cells (Figure 6d) was 61.0 times more efficient compared with translocation of cell-free HIV-1 (Figure 6e), the respective mean±s.e.m. percentage of translocation being 1.22±0.75% and 0.02±0.02% (P=0.0244). Importantly, these results also reveal that HIV-1 translocation is more efficient across inner compared with outer foreskin reconstructions, irrespective of the source of virus (Figure 6d and e). Similar results were also obtained following exposure to cell-free HIV-1 JR-CSF (data not shown).
For both forms of HIV-1 inocula, that is, V29-infected PBMCs and cell-free HIV-1 V29 (Figure 6f and g), HIV-1 translocation was concentration-dependent, and was always higher when induced from HIV-1-infected cells compared with cell-free HIV-1.
This set of results shows that efficient HIV-1 translocation occurs when induced by HIV-1-infected cells and through inner foreskin reconstructions. In contrast, cell-free HIV-1 poorly translocates and outer foreskin reconstructions are much less permissive to HIV-1 translocation.
SP mixed with CVSs decreases HIV-1 translocation through inner foreskin reconstructions
In vivo, during sexual intercourse, HIV-1 is transmitted by genital fluids such as SP and CVS. To evaluate the impact of these fluids on HIV-1 entry at the foreskin, the apical poles of inner foreskin reconstructions were inoculated with HIV-1 V29-infected PBMCs alone or together with various genital fluids, at dilutions of 1:10 for SP and 1:4 for CVS, to mimic their natural dilution during sexual intercourse.48 These dilutions of genital fluids were not toxic to the cells, as judged by cell viability evaluated by trypan blue exclusion (data not shown). To mimic the in vivo events during heterosexual intercourse between uninfected men and HIV-1-infected women, SP from HIV-1-negative men was mixed with CVS from HIV-1-positive women (or from HIV-1-negative women for control). HIV-1 translocation to the basal chamber was evaluated by p24 ELISA 1 h later.
The presence of either SP or CVS alone had no effect on the translocation of HIV-1 induced from HIV-1 V29-infected PBMCs across inner foreskin reconstructions (Figure 6h). In contrast, the mixture of SP and CVS from HIV-1-positive women significantly decreased HIV-1 translocation to the basal chamber (Figure 6h). A similar effect was found for a mixture of SP and CVS from HIV-1-negative women, although the decrease of HIV-1 translocation was smaller. The decreased translocation was not a result of inhibition of virus budding/production, as HIV-1 p24 amounts in the apical chambers were similar in the presence or absence of genital fluids (data not shown). To our knowledge, this is the first demonstration of an interaction between SP and CVS that leads to a decrease in HIV-1 transmission.
Discussion
According to an updated report on the global AIDS epidemic (see http://www.unaids.org), nearly 15 million men are currently infected with HIV-1 worldwide. Although clear evidence now points to the protective effect of circumcision in preventing HIV-1 transmission from women to men,17, 18, 19, 20 the exact mechanisms responsible for HIV-1 acquisition in the male genital tract in general, and in the foreskin epithelium in particular, are to a large extent unknown.
Previous studies have documented the presence of possible HIV-1 target cells in the human foreskin. However, they have reached contradictory results regarding the densities of CD1a+ and CD4+ cells in inner compared with outer foreskin, reporting either a similar density,21 a higher density in outer foreskin,23 or a higher density in inner foreskin.22, 27 These discrepancies may be related to the fact that CD1a and CD4 are not cell-specific markers, therefore complicating the identification and quantification of distinct cell populations. Moreover, in these studies, cellular densities were evaluated in foreskin regions that combined both the epidermal and dermal compartments.
In contrast, in the studies reported here, we performed immunohistochemical staining of inner and outer foreskin with Abs that target cell-specific markers, namely, langerin for LCs, CD3 for T cells, as well as DC-SIGN for DCs and CD68 for macrophages. Respective cell densities were evaluated in either the epidermis or dermis. This approach showed that the inner foreskin is rich in langerin+ LCs and CD3+ T cells (see Figure 1), suggesting (as shown later herein) that the higher densities of LCs and T cells contribute to the increased susceptibility of the inner foreskin to HIV-1.
Interestingly, isolated langerin+ LCs were also detected in the foreskin dermis in a third of all foreskin tissues that we examined (data not shown). Whether these langerin+ cells represent epidermal LCs that have migrated from the epidermis to the dermis or rather a distinct subpopulation of dermal langerin+ cells, as recently identified in mouse skin,49, 50, 51 is currently an open question.
A major problem in investigating mucosal HIV-1 transmission is the lack of suitable in vitro models. Moreover, nonprimates are not susceptible to HIV-1, and experiments with primates are expensive and time elaborating. Thus, two approaches may be applied to overcome these obstacles: the use of mucosal tissue explants ex vivo or the development of novel and relevant mucosal models in vitro.
In previous studies, foreskin/skin tissue explants22, 27 or single LCs emigrating from skin epidermis36, 38, 52 seemed permissive to infection with R5, but not with X4, HIV-1. Skin-derived HIV-1-infected LCs could subsequently transfer the virus to either cocultured or conjugated T cells.36, 38, 52 However, in these studies, HIV-1 infection was not always initiated in a polarized manner, that is, restricted to the apical side as occurs in vivo. Moreover, these studies investigated only infection by high concentrations of cell-free HIV-1, and infection was evaluated in LCs emigrating from skin explants >24 h after exposure to the virus, a time point at which the barrier function of the tissue may no longer be intact.
Alternatively, much progress has been made recently in tissue engineering and in in vitro reconstruction of mucosal epithelia. Seeding human epithelial cells onto various dermal equivalents generates such three-dimensional models of the human oral, vaginal, and skin epithelia, which present the structural and morphological characteristics of their respective normal tissues. Importantly, immunocompetent mucosal equivalents can be produced by integrating LCs/DCs into these reconstructions.29, 53, 54, 55, 56, 57, 58, 59 The integrated cells present their normal features (for example, maturation state, expression of various cell markers) and are retained within the epithelia, in contrast to their rapid emigration out of mucosal tissue explants. LCs/DCs integrated in reconstructed vaginal epithelia were permissive to high loads of cell-free HIV-1.57, 59 Yet, immunocompetent skin reconstructions have never before been used to investigate the possible mechanisms of HIV-1 transmission in the foreskin.
Herein, we developed novel ex vivo explants of inner and outer foreskins adapted for polarized infection by HIV-1 (see Figure 2). Migratory cells, such as LCs and T cells, emigrate out of mucosal explants after few hours (a feature that is routinely used in many studies to collect these cells), thus preventing the testing of their exact role in early HIV-1 transmission. We therefore restricted our investigations using foreskin explants to the first hour after exposure to the virus, a time frame in which migratory cells are still present within the tissue. In parallel, in vitro reconstructed models of the human inner and outer foreskin, which present the major characteristics of normal foreskin and also include LCs/DCs, were also developed (see Figure 5). Using these newly developed models, we investigated the pathways used by HIV-1 to penetrate the foreskin. Importantly, these two novel models are complementary, as foreskin explants are better suited for morphological studies (that is, detection of various HIV-1 target cells, and modifications in their density or distribution within the explants after exposure to HIV-1), whereas foreskin reconstructions permit studies of HIV-1 translocation and screening for various factors that may modify this process.
Our results show that efficient entry/translocation of HIV-1 takes place through inner, but not outer, foreskin. Moreover, the outer foreskin seems less susceptible to HIV-1, as no changes in the percentage or distribution of LCs within the epidermis were observed in outer foreskin explants after 1 h of viral exposure. This suggests that the more keratinized outer foreskin provides a physical barrier to HIV-1 entry, by trapping HIV-1 particles (as indeed shown here), and may possibly prevent the sampling and internalization of the virus by LCs. In contrast, our results show that both the higher density of LCs and T cells and the lower degree of keratinization correlate, and therefore most likely contribute, to the increased susceptibility of the inner foreskin to HIV-1. The less keratinized inner foreskin provides no efficient barrier against HIV-1, enabling virus entry. In line with our observations, skin abrasion was necessary to enable infection of LCs with HIV-1 in previous skin explant studies,36, 38 probably to remove the protective thick keratin layer of normal skin. The reduced permissiveness of the outer foreskin to HIV-1 may also reflect functional differences between LCs in outer and inner foreskin. In addition, different set of cytokines/chemokines could be secreted by each tissue part that could affect LCs mobility, and thereby antigen sampling capacity in inner and outer foreskin. Further studies should focus on factors secreted by inner and outer foreskin tissues in various infection conditions.
We further compared in our studies for the first time the relative infectious potential of cell-free vs. cell-associated HIV-1, and showed that HIV-1 transmission is much more effective when the foreskin is inoculated with HIV-1-infected cells. Indeed, only HIV-1-infected cells induced the formation of LC–T cell conjugates in inner foreskin explants, and translocation of cell-associated HIV-1 across foreskin reconstructions was more efficient compared with that of cell-free HIV-1. Of note, the HIV-1-infected cells themselves did not enter/translocate across either the foreskin explants or the reconstructions, as fluorescently loaded HIV-1-infected cells were never detected within the epithelium or the basal compartment after their inoculation at the apical surface (data not shown). These results may explain why a very high concentration of cell-free HIV-1 was needed in previous studies to detect infection in the tissues or in emigrating LCs.22, 27, 36, 38, 52 Viral synapses formed between HIV-1-infected cells and apical foreskin keratinocytes, which lead to polarized viral budding, were also detected in our studies. This suggests that the newly budded virus, which concentrates locally in the synaptic cleft, may increase virus infectivity, which in turn may enter more efficiently into foreskin explants and induce LC–T cell conjugate formation, or be translocated across foreskin reconstructions.
Importantly, our results further show that the viral load is crucial in determining the outcome of foreskin exposure to HIV-1. Virus-induced LC–T cell conjugate formation in inner foreskin explants, and translocation of HIV-1 across inner foreskin reconstruction, were evident only at high concentrations of the virus. At low concentrations of HIV-1 (either cell-free or cell-associated), LCs are retained in the inner foreskin, probably to sample and degrade the virus, as previously reported.16 In contrast, higher concentrations of the virus overcome the protective barrier capacity of langerin against HIV-1 infection.16 In these conditions, LCs internalize the virus, but instead of degrading it, transfer the virus to T cells across conjugates formed between these two cell types (Figure 4). High concentrations of cell-free HIV-1 could not induce such conjugate formation, suggesting that different molecular events take place after exposure of inner foreskin to highly infected cells or to high concentration of cell-free virus. This suggests that infected cells forming synapses with the foreskin surface may transmit signals to keratinocytes or directly to LCs, thus affecting trafficking of LCs to the mucosal surface and/or the epidermal/dermal interface and conjugation with T cells.
Langerhans cell–T cell conjugates detected in our experiments formed very rapidly, within the first hour of viral exposure. However, this time frame is too short to lead to productive infection of either LCs or T cells with HIV-1, preventing the direct testing of their infection rate in the present experimental settings. In addition, this time period is probably too short to allow migration of the conjugates and/or isolated migratory cells out of the explants into the basal chambers. Accordingly, the contents of the basal chambers were also collected after 1 h of polarized exposure of foreskin explants to either cell-free HIV-1 or HIV-1-infected cells. When added to reporter-activated PBMCs to amplify and quantify the amount of HIV-1 that might have translocated through the explants, no signal was detected (data not shown). Moreover, visual examination of the basal chambers did not reveal the presence of emigrants with a typical LC or T cell morphology (data not shown). These findings suggest that during the very first hour of viral exposure, HIV-1 has the capacity to modify the density/spatial distribution of LCs and T cells within the inner foreskin epidermis. In contrast, HIV-1-mediated emigration of LCs/T cells out of the foreskin may be evident only at later time points.
HIV-1 transmission occurs mainly through sexual intercourse, which involves mixing of genital fluids (for example, SP and CVSs). Thus, in order to mimic these in vivo events, inner foreskin reconstruction was exposed to cell-associated HIV-1 (that is, conditions in which efficient HIV-1 translocation takes part, as we show) in the presence of genital fluids. This experimental approach revealed for the first time the existence of interplay between these fluids that leads to decreased HIV-1 translocation. These novel findings thus suggest that HIV-1 transmission in vivo (in the foreskin in particular, and perhaps also in other mucosal tissues) may be lower than that routinely evaluated in vitro. Previous studies have shown that SP can induce secretion of chemokines by the female genital mucosa, leading to subsequent transient attraction of mononucleated cells,48 which may in turn increase the susceptibility to HIV-1 infection. Two recent studies have investigated the role of SP in HIV-1 infection, but have reached contradictory results, by showing that semen may either amplify HIV-1 infection of T cells or trans-infection of T cells by DCs,33 or inhibit the capture and transfer of HIV-1 by DCs.32 In fact, the role of genital fluids in models of HIV-1 infection is rarely evaluated, and the exact factors in such fluids that may increase or decrease HIV-1 sexual transmission are mostly undefined. Thus, further studies are needed to clearly identify these factors and their contribution to HIV-1 infection. Identification of these molecular factors in genital fluids that contribute to HIV-1 transmission may be of clinical importance, and may be used to develop novel anti-HIV-1 agents.
On the basis of the present study, we propose, as schematized in Figure 7, that the main pathway for HIV-1 entry at the foreskin results from interaction of HIV-1-infected cells with the inner part of the foreskin. The inner foreskin might be exposed to HIV-1+ cells either during intercourse or later on after foreskin retraction that may trap HIV-1+ cells. Such contact would lead to viral synapse formation, resulting in HIV-1 budding and capture by epidermal LCs. In turn, within 1 h, LCs loaded with HIV-1 would rapidly migrate to the epidermal/dermal interface, form conjugates with T cells, and transfer the virus to T cells. Such infected T cells might initiate the spread of the infection. Molecular signals, such as chemokines that could be induced by HIV-1 within the foreskin tissue and that may drive LCs migration to the mucosal surface and/or to the dermal compartment, are currently under investigation. Together, our results rationalize at the cellular level the apparent protective outcome of circumcision against HIV-1 acquisition by men: both surfaces of the foreskin are invaded by HIV-1, especially on contact with HIV-1-infected cells. Thus, removal of the foreskin (and especially the inner aspect) after circumcision results in the elimination of a mucosal surface rich in HIV-1 target cells (for example, LCs and T cells), which serves as an HIV-1 entry portal in men.
Schematization of the initial events occurring in the foreskin during human immunodeficiency virus type-1 (HIV-1) transmission. (Left) In inner foreskin, HIV-1-infected cells form viral synapses resulting in HIV-1 particle budding (1). Low concentration of HIV-1, either from cell-free inoculums or budded from cells weakly infected with HIV-1, induces retention of Langerhans cells (LCs) within the epidermis, and results in virus internalization and degradation by LCs (2). In contrast, cells highly infected with HIV-1 (3) attract LCs to the mucosal surface, which capture HIV-1 (4) and then migrate back to the epidermal–dermal interface. There, LCs form conjugates with T cells allowing HIV-1 transfer to T cells (5). We speculate that this process may lead either to emigration of HIV-1-positive LC–T cell conjugates and/or isolated T cells, and potentially also to transfer/emigration of dermal dendritic cells (DCs) that in turn would disseminate the infection (6). High loads of cell-free HIV-1 remain inefficient at LC–T cell conjugate formation. (Right) In outer foreskin, HIV-1-infected cells also form viral synapses with the apical foreskin surface (7). However, both newly budded HIV-1 particles and cell-free HIV-1 virions remain trapped within the thick layer of keratin at the mucosal surface (8). Thus, virus entry into the epidermis is limited, and infection may not be disseminated (9).
Methods
Tissue and genital fluids
Normal foreskin tissues were obtained from the Geoffroy Saint-Hillaire Clinic and the Urology Service at the Necker Hospital in Paris, France, from healthy adults (mean age 42 years old, range 17–87 years) undergoing circumcision due to personal reasons or phimosis. Foreskin tissues removed because of cancerous or infectious pathologies were discarded and never used in the current study. Informed consents were obtained from all individuals. Tissues were placed in phosphate-buffered saline (PBS) supplemented with 20 μg ml−1 gentamicin (Gibco Invitrogen, Carlsbad, CA), transported to the laboratory immediately after circumcision, and processed within the next 2 h. Foreskin tissues were separated mechanically into inner and outer parts, distinguished by their different colors and morphology, and any remaining fat and muscle tissue was removed from the dermal side.
Cervicovaginal secretions from HIV-1 seropositive women were collected at the Pasteur Institute from Phnom Penh, Cambodia, as previously described,4 following approval by the local ethics committee. Informed consents were obtained from all individuals. For genital secretions, ethics approval was obtained from the Cambodian National Ethics Committee. CVSs from HIV-1 seronegative women and SP from HIV-1 seronegative men were obtained from individuals attending the Reproductive Biology Service at the Cochin hospital, Paris, France (following approval by the Cochin Hospital ethical Person Protection committee (Comité de Protection des Personnes de Cochin)). Informed consents were obtained from all individuals. All samples were centrifuged, aliquoted, frozen, and kept at −80°C for further use.
Ex vivo polarized foreskin tissue explants
Round pieces of foreskin tissue, from either inner or outer foreskin, were cut using a 8 mm Harris Uni-Core, and placed with their epidermal side facing up on top of a polycarbonate membrane (12 μm pore size, 12 mm diameter) of a two-chamber Costar Transwell permeable insert (Corning, Corning, NY). Hollow plastic cloning ring cylinders of 6 mm inner diameter (VMR, Strasbourg, France) were glued to the apical surface of each tissue piece, using the two-component biological fibrin sealant Tissucol kit (Baxter, Vienna, Austria) that is used clinically for tissue sealing during surgery.60 A 10 μl drop of 4 U ml−1 thrombin solution was mixed with a 10 μl drop of fibrinogen solution (both solutions prepared according to the manufacturer's instructions), which simulates the key features of the physiological coagulation process. The mixture was rapidly applied to one side of each cloning ring, which was then pressed gently with fine forceps onto the apical surface of the tissue for 10 s, permitting the cylinders to be firmly glued to the apical surface of each tissue explant and resulting in the creation of a sealed and polarized apical chamber.
Firm attachment of the cylinders was evident by their retention on the epidermal surface, even when the two-chamber system inserts were inverted to an upright position. Furthermore, to verify that the cylinder-based apical chambers are indeed completely sealed, 100 μl of either RPMI 1640 medium or trypan blue solution (Gibco) was added to the inner space of the cylinders, and the explants were incubated for 1–4 h at 37°C. After incubation, no signs of leakage were detected, as evident by the ability to completely recover the total volume of medium initially added and the complete absence of trypan blue traces around the cylinder edges or in the basal chamber.
Purification of primary foreskin cells
Tissues were cut into 5 × 5 mm2 pieces that were incubated with their epidermal side facing up in 100 mm culture dishes containing 10 ml RPMI 1640 medium (Gibco) supplemented with 2.4 U ml−1 Dispase II (Roche Diagnostics GmbH, Mannheim, Germany) overnight at 4°C. The epidermis was then separated from the dermis using forceps.
To purify epidermal keratinocytes, the epidermal sheets were incubated in 5 ml 0.05% Trypsin/EDTA (Gibco) for 10 min at 37°C. Trypsin was then inactivated with 5 ml fetal bovine serum (FBS; PAN Biotech GmbH, Aidenbach, Germany), and a single-cell suspension was obtained by mechanical disruption using a 10 ml pipette, followed by filtration through a 40 μm nylon cell strainer and centrifugation. The resulting keratinocytes were grown in keratinocyte serum-free medium containing L-glutamine, supplemented with 25 μg ml−1 bovine pituitary extract, 0.25 ng ml−1 epidermal growth factor, 5 μg ml−1 gentamicin, and 5 μg ml−1 amphotericin B (Gibco). To purify dermal fibroblasts, the dermal pieces were incubated in 2.5 ml RPMI 1640 medium supplemented with 2 mg ml−1 collagenase type IV-S (Sigma, St Louis, MO) and 200 U ml−1 DNase I (Roche) for 2 h at 37°C. After inactivation with 5 ml FBS, tissue pieces were disrupted by vortexing for 30 s, and the resulting cell suspension was filtered through a 100 μm nylon cell strainer and centrifuged. The resulting fibroblasts were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 2 mM L-glutamine, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin (Gibco).
For both cell types, culture media were changed three times a week. Cells were grown to confluence, split with Trypsin/EDTA, and second passage cells were aliquoted and stored at −80°C for further use. Keratinocytes were only used at passages 2–4 and fibroblasts at passages 2–7.
Purification of PBMCs and preparation of DCs and LCs
Peripheral blood mononuclear cells from healthy donors were separated from whole blood by a standard Ficoll gradient. CD14+ monocytes were obtained from PBMCs using a CD14-negative magnetic selection kit (Stemcell Technologies, Grenoble, France), according to the manufacturer's instructions, and were cultured (1 × 106 cells per 1 ml per 12-well plate) in RPMI 1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin. DCs and LCs were generated from CD14+ monocytes by adding 10 ng ml−1 IL-4 and 100 ng ml GM-CSF (Peprotech, Rocky Hill, NJ) for DCs, as well as 10 ng ml−1 TGFβ1 (R&D systems, Minneapolis, MN) for LCs, as previously described.61 On days 2 and 4 of culture, 0.5 ml fresh medium supplemented with the same cytokines at a similar concentration was added to each well. The resulting cells were used at day 7 of culture. Alternatively, a mixed population of DCs/LCs was obtained by culturing CD14+ monocytes in a similar manner in medium supplemented with 10 ng ml−1 IL-13 (R&D systems), 200ng ml−1 GM-CSF, and 10 ng ml−1 TGFβ1, as described.62
Virus and infected PBMCs
Virus was obtained from the NIH AIDS reagent program. The HIV-1 primary isolate 93BR029 (clade B, R5 tropic, termed herein as V29) was amplified on phytohemagglutinin-stimulated PBMCs as described.63 The cell-free HIV-1 JR-CSF molecular clone (clade B, R5 tropic) was produced by transfection of HEK293 T cells with proviral DNA as described.64 HIV-1 p24 antigen was quantified by the p24 Innotest HIV-1 ELISA (Innogenetics, Gent, Belgium), and viral stocks were aliquoted for single use and stored at −80°C. Two doses of cell-free HIV-1 were tested, that is either 20 or 100 ng of p24, referred to in the text as low and high cell-free HIV-1 loads, respectively.
Peripheral blood mononuclear cells were infected with V29 as described.63 Briefly, phytohemagglutinin/IL-2-stimulated PBMCs (5 × 106 cells in 10 ml RPMI 1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, and 10 U ml−1 IL-2 (Roche)) were inoculated with either 60 or 200 ng p24 of HIV-1 V29. After 2 days, further noninfected phytohemagglutinin/IL-2-stimulated cells were added (20 × 106 cells in 10 ml of the same medium containing 20 U ml−1 IL-2). Infection was monitored in culture supernatants by p24 ELISA and by p24 intracellular staining and flow cytometry analysis. Infected cells were used between days 7 and 14 after addition of virus. A total of 1 × 106 cells, infected either with low or high doses of HIV-1 V29, released 20 ng and 100 ng p24, respectively, after 1 h incubation at 37°C and are referred to in the text as PBMCs weakly or highly infected with HIV-1 V29. In addition, 1–2% and 5–7% of the PBMCs weakly and highly infected with HIV-1 V29 were positive for p24, respectively, as evaluated by p24 intracellular staining.
In vitro foreskin reconstruction
Foreskin fibroblasts (from either inner or outer foreskin) were seeded on top of a polycarbonate membrane (3 μm pore size) of a two-chamber 12-mm diameter Costar Transwell insert (2–5 × 105 cells per insert), and were grown under submerged conditions (0.5 and 1 ml medium in apical and basal chambers, respectively) in a medium that was previously reported to promote the formation of a dermis-like collagen-rich fibrous sheet.42 This medium consisted of a 3:1 mixture of DMEM and Ham's nutrient mixture F12 with L-glutamine (Gibco), supplemented with 10% FBS, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 5 ng ml−1 epidermal growth factor (Invitrogen), 5 μg ml−1 insulin (Gibco), 0.4 μg ml−1 hydrocortisone, 5 μg ml−1 transferrin, and 10 pM Triiodothyronine (Sigma). In addition, 5 mg ml−1 ascorbic acid (Sigma) was added fresh at each medium change (three times a week). After 3 weeks of submerged culture, foreskin keratinocytes (from either inner or outer foreskin) were seeded on top of the fibrous sheet (2–5 × 105 cells per insert). LCs/DCs (0.5–1 × 105 cells per insert, a mixture of cells differentiated alone or obtained as a mixed population, see above) were added twice, first together with the keratinocytes and then alone, 1 week later. Reconstructions containing keratinocytes and LCs/DCs were cultured in the same medium described above, but from which epidermal growth factor was eliminated. Inner foreskin reconstructions were further cultured for 2 weeks under submerged conditions, and then for 2 additional weeks at the air–liquid interface (0.5 ml of the medium in basal chamber only). Outer foreskin reconstructions were cultured for 4 weeks at the air–liquid interface.
Short-term polarized infection assay
Foreskin explants and reconstructions were exposed apically and in a polarized manner (by adding 100 μl into the inner space of the cloning ring cylinders adhered to the tissue explants or 200 μl to the apical chamber of the foreskin reconstructions) to either HIV-1 V29-infected PBMCs or cell-free HIV-1 (V29 or JR-CSF). Noninfected PBMCs or RPMI 1640 medium alone served as negative controls. Lower chambers were supplemented with 0.5 ml RPMI 1640 medium. In some experiments, foreskin reconstructions were exposed to V29-infected PBMCs in the presence of SP from HIV-1 seronegative men (diluted 1:10) and CVSs from HIV-1 seropositive/seronegative women (diluted 1:4), either alone or mixed together. After 1 h incubation at 37°C, the content of both apical and basal chambers was removed, centrifuged, and the supernatant fractions were aliquoted and stored at −80°C for further quantification of HIV-1 by p24 ELISA. The explants/reconstructions were then fixed in 4% paraformaldehyde for further morphological evaluation. In other experiments, foreskin explants were processed for flow cytometry analysis (see below).
Preparation of epidermal and dermal single-cell suspensions for flow cytometry
Triplicate foreskin explants were inoculated with HIV-1 as described above, and after removal of the cloning ring cylinders, pooled tissue pieces were incubated with their epidermal side facing up in 1 ml RPMI 1640 medium supplemented with 2.4 U ml−1 Dispase II (in a 12-well plate) overnight at 4°C, after which epidermis and dermis were mechanically separated using forceps.
Epidermal single-cell suspensions were prepared by incubating pooled epidermal sheets from each triplicate in 1 ml of 0.025% Trypsin/EDTA (Clonetics, Cambrex Bio Science, Walkersville, MD) for 10 min at 37°C, followed by inactivation of trypsin with 1 ml FBS, mechanical disruption using a 5 ml pipette, filtration of released cells through a 40-μm nylon cell strainer, and centrifugation. In some experiments, after similar Dispase treatment, single-cell suspensions were prepared from epidermal sheets derived from normal foreskin tissues.
Dermal single-cell suspensions were prepared by incubating pooled dermal pieces from each triplicate in 1 ml RPMI 1640 medium supplemented with 2 mg ml−1 Collagenase and 200 U ml−1 DNase for 2 h at 37°C, followed by inactivation with 1 ml FBS, tissue disruption by vortexing for 30 s, filtration through a 100-μm nylon cell strainer, and centrifugation.
Flow cytometry
For immunofluorescent staining of surface CD3, CCR5, langerin, and DC-SIGN in epidermal and/or dermal cell suspensions (prepared from HIV-1-infected or control foreskin explants, as well as normal foreskins), cells were resuspended in PBS (devoid of Ca2+/Mg2+ ions) supplemented with 2% FBS and 1 mM EDTA (that is, staining buffer), and transferred to a round-bottomed 96-well plate (0.1–0.5 × 106 cells per well). For single staining, cells were incubated for 30 min on ice with 5–10 μg ml−1 of APC-conjugated mouse-anti-human mAbs to CD3 (BD Pharmingen, San Jose, CA) or DC-SIGN (R&D systems). For double staining, cells were incubated for 30 min on ice with 5–10 μg ml−1 of APC-conjugated mouse-anti-human mAbs to either CD3 or langerin (R&D systems) and FITC-conjugated mouse-anti-human CCR5 mAb (BD Pharmingen). All Abs were diluted in staining buffer to a final volume of 50 μl per well. Cells were then washed with staining buffer, centrifuged, and fixed for 15 min at room temperature with 4% paraformaldehyde.
For immunofluorescent staining of intracellular langerin, paraformaldehyde-fixed cells were permeabilized for 15 min at room temperature with PBS containing 0.1% saponin, and then incubated for 30 min at room temperature with 5–10 μg ml−1 of phycoerythrin-conjugated mouse-anti-human langerin mAb (Beckman, Marseille, France), diluted in PBS/0.1% saponin to a final volume of 50 μl per well.
Cells stained with matched isotype control Abs served as negative controls. Fluorescence profiles were recorded using a FACSCalibure (BD Biosciences, Rungis, France) and results were analyzed using the CellQuest Pro software (BD Biosciences, Rungis, France).
Immunofluorescent microscopy and immunohistochemistry
After infection and fixation, foreskin tissue explants and insert-grown foreskin reconstructions were embedded in paraffin, and serial 4-μm sections were cut. Sections were deparaffinized in xylene and graded alcohol solutions, microwave heated in 10 mM citrate buffer (pH=6.0) for antigen retrieval, cooled down in the same buffer, and washed in PBS.
For immunofluorescent and confocal microscopy, sections were blocked in PBS containing 20% horse serum (Vector Laboratories, Burlingame, CA) and 0.1% tween (Sigma) for 1 h at room temperature. After several washes in PBS, sections were incubated overnight at 4°C with primary Abs (50 μl per section, diluted in PBS/2% horse serum/0.1% tween), including mouse-IgG3-anti-human CK14 at 1 μg ml−1 (Labvision, Fremont, CA), mouse-IgG1-anti-human CK10 at 0.5 μg ml−1 (DakoCytomation, Glostrup, Denmark) goat-IgG-anti-human langerin at 20 μg ml−1 (R&D systems), human serum positive for anti-HIV-1 Abs at 1:100 dilution (positive control from the New Lav Blot HIV-1 kit; Bio-Rad Laboratories, Marnes-la-Coquette, France), the human mAb 2F5 that recognized HIV-1 gp41 at 5 μg ml−1 (NIH, AIDS reagent program), and a cocktail of several mouse and human mAbs (5–10 μg ml−1) recognizing HIV-1 gp41/gp120/gp160/p24: D50 (NIH), 2G12 (NIH), 41A (Hybridolab, Pasteur Institute, Paris, France) and Kal-1 (Dako). Sections were then washed with PBS and incubated for 1 h at room temperature with appropriate secondary Abs (50 μl per section, diluted in the ratio 1:50 to 1:100 in PBS), including FITC-conjugated anti-mouse-IgG3 (SouthernBiotech, Birmingham, AL), TRITC (tetramethyl rhodamine isothiocyanate)-conjugated anti-mouse-IgG1 (SouthernBiotech), FITC-conjugated anti-goat-IgG (Jackson Immunoresearch, West Grove, PA), TRITC-conjugated anti-human IgG (Jackson Immunoresearch), FITC-conjugated anti-human IgG (Jackson Immunoresearch), and FITC-conjugated anti-mouse-IgG (Jackson Immunoresearch). Cell nuclei were then stained with 5 μg ml−1 DAPI (4′,6-diamidino-2-phenylindole; Sigma; 50 μl per section, 10 min at room temperature), and the sections were washed again in PBS and mounted with MOWIOL medium (Calbiochem Merck Darmstadt, Germany).
For immunohistochemistry, the LSAB2 System-HRP (DakoCytomation) was used according to the manufacturer′s instructions. All Abs, including mouse-IgG2b-anti-human langerin (Abcam, Cambridge, UK), mouse-IgG1-anti-human CD3 (Dako), mouse-IgG2b-anti-human DC-SIGN (R&D systems), mouse-IgG1-anti-human CD68 (Dako), mouse-IgG1-anti-human CD1a (Immunotech, Marseille, France), and mouse-IgG1 anti-human involucrin (Sigma) were diluted to 10 μg ml−1 in Ab diluent (Dako), incubated with tissue sections for 10–30 min at room temperature (50 μl per section), and visualized with either AEC red (3-amino-9-ethylcarbazole) or DAB brown (di-amino benzidine) substrates. Sections were counterstained for 30 s with hematoxyline solution (Dako) and mounted as described above. In some experiments, Masson's trichrome staining was performed according to standard techniques.
For immunofluorescent microscopy and immunohistochemistry, images were taken using an × 40 objective of a Nikon E600 microscope (Nikon, Tokyo, Japan) equipped with a CCD QICAM camera (QImaging, Surrey, BC, Canada). For cell quantification, the ImageJ software (NIH) was used to count the number of positively stained cells in a minimum of 10 separate fields and calculate the surface of either the epidermis or dermis in each field. Measurement of the distance between LCs and the mucosal surface was evaluated using the Arcgis V9.3 program (ESRI, Redlands, CA) analysis. Distances of 183 (inner, noninfected), 166 (inner, infected), 67 (outer, noninfected), and 163 (outer, infected) cells were measured and statistically analyzed (see http://resources.esri.com/help/9.3/ArcGISDesktop/com/Gp_ToolRef/coverage_tools/near_coverage_.htm). For confocal microscopy, staining was visualized with a Leica TCS SP spectral microscope (Leica Microsystems, Wetzlar, Germany), and acquired image stacks were analyzed with Imaris Software (Bitplane AG, Zurich, Switzerland).
Electron microscopy
Foreskin explants or reconstructions were fixed for 1 h with 3% glutaraldehyde. Samples were then postfixed in 1% osmium tetroxide in 0.1 M PBS and dehydrated in increasing graded alcochol solutions. After 10 min incubation in a mixture of 1:2 epoxy propane and epoxy resin, the samples were embed in gelatine capsules with freshly prepared epoxy resin and polymerized at 60°C for 24 h. Sections (80 nm) were then cut with an ultramicrotome (Reichert Ultracut S, Reichert Technologies, Depew, NY), stained with uranyl acetate and Reynold's lead citrate, and observed with a transmission electron microscope (JEOL 1011, JEOL, Tokyo, Japan), equipped with a GATAN numerical camera (Gatan, Munich, Germany). Pictures were taken and digitalized with Digital Micrograph software (Gatan, Munich, Germany).
Statistical analysis
Statistical significance was analyzed by the Student's t-test. For comparing HIV-1 translocation in inner foreskin reconstructions in the presence of different genital fluids, pair-wise comparisons were performed by the nonparametric Mann–Whitney U-test.
References
Ross, H.M., Romrell, L.J. & Kaye, G.I. Histology, A Text An Atlas Third edn. ( Williams & Wilkins, Baltimore, 1995 ).
Bomsel, M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 3, 42–47 (1997).
Bomsel, M. et al. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 9, 277–287 (1998).
Alfsen, A., Iniguez, P., Bouguyon, E. & Bomsel, M. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J. Immunol. 166, 6257–6265 (2001).
Alfsen, A., Yu, H., Magerus-Chatinet, A., Schmitt, A. & Bomsel, M. HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol. Biol. Cell 16, 4267–4279 (2005).
Meng, G. et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat. Med. 8, 150–156 (2002).
Tan, X., Pearce-Pratt, R. & Phillips, D.M. Productive infection of a cervical epithelial cell line with human immunodeficiency virus: implications for sexual transmission. J. Virol. 67, 6447–6452 (1993).
Pearce-Pratt, R. & Phillips, D.M. Studies of adhesion of lymphocytic cells: implications for sexual transmission of human immunodeficiency virus. Biol. Reprod. 48, 431–445 (1993).
Hubner, W. et al. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science 323, 1743–1747 (2009).
Phillips, D.M. The role of cell-to-cell transmission in HIV infection. AIDS 8, 719–731 (1994).
Merad, M., Ginhoux, F. & Collin, M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 8, 935–947 (2008).
Hladik, F. & McElrath, M.J. Setting the stage: host invasion by HIV. Nat. Rev. Immunol. 8, 447–457 (2008).
de Witte, L., Nabatov, A. & Geijtenbeek, T.B. Distinct roles for DC-SIGN+-dendritic cells and Langerhans cells in HIV-1 transmission. Trends Mol. Med. 14, 12–19 (2008).
Figdor, C.G., van Kooyk, Y. & Adema, G.J. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2, 77–84 (2002).
Turville, S.G. et al. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3, 975–983 (2002).
de Witte, L. et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 13, 367–371 (2007).
Quinn, T.C. Circumcision and HIV transmission. Curr. Opin. Infect. Dis. 20, 33–38 (2007).
Auvert, B. et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2, e298 (2005).
Bailey, R.C. et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 369, 643–656 (2007).
Gray, R.H. et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 369, 657–666 (2007).
Hussain, L.A. & Lehner, T. Comparative investigation of Langerhans′ cells and potential receptors for HIV in oral, genitourinary and rectal epithelia. Immunology 85, 475–484 (1995).
Patterson, B.K. et al. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am. J. Pathol. 161, 867–873 (2002).
McCoombe, S.G. & Short, R.V. Potential HIV-1 target cells in the human penis. AIDS 20, 1491–1495 (2006).
Pask, A.J., McInnes, K.J., Webb, D.R. & Short, R.V. Topical oestrogen keratinises the human foreskin and may help prevent HIV infection. PLoS ONE 3, e2308 (2008).
Donoval, B.A. et al. HIV-1 target cells in foreskins of African men with varying histories of sexually transmitted infections. Am. J. Clin. Pathol. 125, 386–391 (2006).
Soilleux, E.J. & Coleman, N. Expression of DC-SIGN in human foreskin may facilitate sexual transmission of HIV. J. Clin. Pathol. 57, 77–78 (2004).
Fischetti, L., Barry, S.M., Hope, T.J. & Shattock, R.J. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. AIDS 23, 319–328 (2009).
Shattock, R.J., Griffin, G.E. & Gorodeski, G.I. In vitro models of mucosal HIV transmission. Nat. Med. 6, 607–608 (2000).
Bouschbacher, M. et al. Early events in HIV transmission through a human reconstructed vaginal mucosa. AIDS 22, 1257–1266 (2008).
Collins, K.B., Patterson, B.K., Naus, G.J., Landers, D.V. & Gupta, P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med. 6, 475–479 (2000).
Zussman, A., Lara, L., Lara, H.H., Bentwich, Z. & Borkow, G. Blocking of cell-free and cell-associated HIV-1 transmission through human cervix organ culture with UC781. AIDS 17, 653–661 (2003).
Sabatte, J. et al. Human seminal plasma abrogates the capture and transmission of human immunodeficiency virus type 1 to CD4+ T cells mediated by DC-SIGN. J. Virol. 81, 13723–13734 (2007).
Munch, J. et al. Semen-derived amyloid fibrils drastically enhance hiv infection. Cell 131, 1059–1071 (2007).
Bourinbaiar, A.S. & Phillips, D.M. Transmission of human immunodeficiency virus from monocytes to epithelia. J. Acquir. Immune Defic. Syndr. 4, 56–63 (1991).
Anderson, D.J. et al. Quantitation of mediators of inflammation and immunity in genital tract secretions and their relevance to HIV type 1 transmission. AIDS Res. Hum. Retroviruses 14 (Suppl 1), S43–S49 (1998).
Kawamura, T. et al. Significant virus replication in Langerhans cells following application of HIV to abraded skin: relevance to occupational transmission of HIV. J. Immunol. 180, 3297–3304 (2008).
Pope, M. et al. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78, 389–398 (1994).
Reece, J.C. et al. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J. Exp. Med. 187, 1623–1631 (1998).
Sugaya, M., Lore, K., Koup, R.A., Douek, D.C. & Blauvelt, A. HIV-infected Langerhans cells preferentially transmit virus to proliferating autologous CD4+ memory T cells located within Langerhans cell-T cell clusters. J. Immunol. 172, 2219–2224 (2004).
Hladik, F. et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26, 257–270 (2007).
MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 445, 874–880 (2007).
Lee, D.Y. et al. A new dermal equivalent: the use of dermal fibroblast culture alone without exogenous materials. J. Dermatol. Sci. 43, 95–104 (2006).
Prunieras, M., Regnier, M. & Woodley, D. Methods for cultivation of keratinocytes with an air-liquid interface. J. Invest. Dermatol. 81, 28s–33s (1983).
Rosdy, M. & Clauss, L.C. Terminal epidermal differentiation of human keratinocytes grown in chemically defined medium on inert filter substrates at the air-liquid interface. J. Invest. Dermatol. 95, 409–414 (1990).
Goto, T., Nakai, M. & Ikuta, K. The life-cycle of human immunodeficiency virus type 1. Micron 29, 123–138 (1998).
Roingeard, P. Viral detection by electron microscopy: past, present and future. Biol. Cell 100, 491–501 (2008).
Perotti, M.E., Tan, X. & Phillips, D.M. Directional budding of human immunodeficiency virus from monocytes. J. Virol. 70, 5916–5921 (1996).
Sharkey, D.J., Macpherson, A.M., Tremellen, K.P. & Robertson, S.A. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol. Hum. Reprod. 13, 491–501 (2007).
Ginhoux, F. et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 204, 3133–3146 (2007).
Bursch, L.S. et al. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 204, 3147–3156 (2007).
Poulin, L.F. et al. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J. Exp. Med. 204, 3119–3131 (2007).
Kawamura, T. et al. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J. Exp. Med. 192, 1491–1500 (2000).
Regnier, M., Staquet, M.J., Schmitt, D. & Schmidt, R. Integration of Langerhans cells into a pigmented reconstructed human epidermis. J. Invest. Dermatol. 109, 510–512 (1997).
Guironnet, G. et al. Phenotypic and functional outcome of human monocytes or monocyte-derived dendritic cells in a dermal equivalent. J. Invest. Dermatol. 116, 933–939 (2001).
Facy, V., Flouret, V., Regnier, M. & Schmidt, R. Reactivity of Langerhans cells in human reconstructed epidermis to known allergens and UV radiation. Toxicol. In vitro 19, 787–795 (2005).
Bechetoille, N. et al. Effects of solar ultraviolet radiation on engineered human skin equivalent containing both Langerhans cells and dermal dendritic cells. Tissue Eng. 13, 2667–2679 (2007).
Sivard, P. et al. HIV-1 infection of Langerhans cells in a reconstructed vaginal mucosa. J. Infect. Dis. 190, 227–235 (2004).
Sivard, P. et al. In vitro reconstructed mucosa-integrating Langerhans′ cells. Exp. Dermatol. 12, 346–355 (2003).
Dumont, S. et al. When integrated in a subepithelial mucosal layer equivalent, dendritic cells keep their immature stage and their ability to replicate type R5 HIV type 1 strains in the absence of T cell subsets. AIDS Res. Hum. Retroviruses 20, 383–397 (2004).
Jackson, M.R. Fibrin sealants in surgical practice: an overview. Am. J. Surg. 182, 1S–7S (2001).
Geissmann, F. et al. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J. Exp. Med. 187, 961–966 (1998).
Bechetoille, N., Andre, V., Valladeau, J., Perrier, E. & Dezutter-Dambuyant, C. Mixed Langerhans cell and interstitial/dermal dendritic cell subsets emanating from monocytes in Th2-mediated inflammatory conditions respond differently to proinflammatory stimuli. J. Leukoc. Biol. 80, 45–58 (2006).
Lagaye, S. et al. Cell-to-cell contact results in a selective translocation of maternal human immunodeficiency virus type 1 quasispecies across a trophoblastic barrier by both transcytosis and infection. J. Virol. 75, 4780–4791 (2001).
Magerus-Chatinet, A. et al. Galactosyl ceramide expressed on dendritic cells can mediate HIV-1 transfer from monocyte derived dendritic cells to autologous T cells. Virology 362, 67–74 (2007).
Acknowledgements
We would like to thank Dr B Weksler (Cornell University, New York, USA) for English editing, as well as C Lesaffre and M Aline for excellent assistance in tissue processing and sectioning for microscopy. Y.G. was supported by Chateaubriand, EMBO and SIDACTION fellowships and M.B. was supported by grants from Agence National de Recherche sur le SIDA et les Hépatites (ANRS), SIDACTION, Fondation pour la Recherche Médicale (FRM), and the European NGIN project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Rights and permissions
About this article
Cite this article
Ganor, Y., Zhou, Z., Tudor, D. et al. Within 1 h, HIV-1 uses viral synapses to enter efficiently the inner, but not outer, foreskin mucosa and engages Langerhans–T cell conjugates. Mucosal Immunol 3, 506–522 (2010). https://doi.org/10.1038/mi.2010.32
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2010.32
This article is cited by
-
HIV transmitting mononuclear phagocytes; integrating the old and new
Mucosal Immunology (2022)
-
CGRP inhibits human Langerhans cells infection with HSV by differentially modulating specific HSV-1 and HSV-2 entry mechanisms
Mucosal Immunology (2022)
-
HIV-1 subverts the complement system in semen to enhance viral transmission
Mucosal Immunology (2021)
-
Human anogenital monocyte-derived dendritic cells and langerin+cDC2 are major HIV target cells
Nature Communications (2021)
-
Langerhans cells in hypospadias: an analysis of Langerin (CD207) and HLA-DR on epidermal sheets and full thickness skin sections
BMC Urology (2019)