Abstract
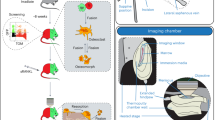
The bone marrow (BM) undergoes extensive remodeling following irradiation damage. A crucial part of restoring homeostasis following irradiation is the ability of hematopoietic stem cells (HSCs) to home to and engraft specialized niches within the BM through a remodeling BM vascular system. Here we show that a combination of ultra-high-field strength magnetic resonance imaging (17.6 T, MRI) coupled with fluorescent microscopy (FLM) serves as a powerful tool for the in vivo imaging of cell homing within the BM. Ultra-high-field MRI can achieve high-resolution three-dimensional (3D) images (28 × 28 × 60 μm3) of the BM in live mice, sufficient to resolve anatomical changes in BM microstructures attributed to radiation damage. Following intra-arterial infusion with dsRed-expressing BM cells, labeled with superparamagnetic iron oxides, both FLM and MRI could be used to follow initial homing and engraftment of donor HSC to a limited number of preferred sites within a few cell diameters of the calcified bone—the endosteal niche. Subsequent histology confirmed the fidelity and accuracy of MRI to create non-invasive, high-resolution 3D images of donor cell engraftment of the BM in living animals at the level of single-cell detection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Till JE, Mc CE . A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 1961; 14: 213–222.
Wilson A, Oser GM, Jaworski M, Blanco-Bose WE, Laurenti E, Adolphe C et al. Dormant and self-renewing hematopoietic stem cells and their niches. Ann N Y Acad Sci 2007; 1106: 64–75.
Schofield R . The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978; 4: 7–25.
Moore KA, Lemischka IR . Stem cells and their niches. Science 2006; 311: 1880–1885.
Kiel MJ, Morrison SJ . Maintaining hematopoietic stem cells in the vascular niche. Immunity 2006; 25: 862–864.
Wilson A, Trumpp A . Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 2006; 6: 93–106.
Taichman RS . Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 2005; 105: 2631–2639.
Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ . SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005; 121: 1109–1121.
Nilsson SK, Johnston HM, Coverdale JA . Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood 2001; 97: 2293–2299.
Chute JP . Stem cell homing. Curr Opin Hematol 2006; 13: 399–406.
Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 2009; 457: 92–96.
Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature 2009; 457: 97–101.
Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci USA 2004; 101: 221–226.
Lin Y, Molter J, Lee Z, Gerson SL . Bioluminescence imaging of hematopoietic stem cell repopulation in murine models. Methods Mol Biol 2008; 430: 295–306.
Bengtsson NE, Brown G, Scott EW, Walter GA . lacZ as a genetic reporter for real-time MRI. Magn Reson Med 2010; 63: 745–753.
Kraitchman DL, Bulte JW . Imaging of stem cells using MRI. Basic Res Cardiol 2008; 103: 105–113.
Daldrup-Link HE, Rudelius M, Piontek G, Metz S, Brauer R, Debus G et al. Migration of iron oxide-labeled human hematopoietic progenitor cells in a mouse model: in vivo monitoring with 1.5-T MR imaging equipment. Radiology 2005; 234: 197–205.
Shapiro EM, Sharer K, Skrtic S, Koretsky AP . In vivo detection of single cells by MRI. Magn Reson Med 2006; 55: 242–249.
Proulx ST, Kwok E, You Z, Papuga MO, Beck CA, Shealy DJ et al. Elucidating bone marrow edema and myelopoiesis in murine arthritis using contrast-enhanced magnetic resonance imaging. Arthritis Rheum 2008; 58: 2019–2029.
Arbab AS, Yocum GT, Kalish H, Jordan EK, Anderson SA, Khakoo AY et al. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood 2004; 104: 1217–1223.
Arbab AS, Yocum GT, Rad AM, Khakoo AY, Fellowes V, Read EJ et al. Labeling of cells with ferumoxides–protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed 2005; 18: 553–559.
Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 2002; 8: 607–612.
Sugimura H, Kisanuki A, Tamura S, Kihara Y, Watanabe K, Sumiyoshi A . Magnetic resonance imaging of bone marrow changes after irradiation. Invest Radiol 1994; 29: 35–41.
Narayan K, Juneja S, Garcia C . Effects of 5-fluorouracil or total-body irradiation on murine bone marrow microvasculature. Exp Hematol 1994; 22: 142–148.
Mazo IB, Gutierrez-Ramos JC, Frenette PS, Hynes RO, Wagner DD, von Andrian UH . Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med 1998; 188: 465–474.
Askenasy N, Farkas DL . Antigen barriers or available space do not restrict in situ adhesion of hemopoietic cells to bone marrow stroma. Stem Cells 2002; 20: 80–85.
Askenasy N, Zorina T, Farkas DL, Shalit I . Transplanted hematopoietic cells seed in clusters in recipient bone marrow in vivo. Stem Cells 2002; 20: 301–310.
Yamashita T, Nabeshima Y, Noda M . High-resolution micro-computed tomography analyses of the abnormal trabecular bone structures in klotho gene mutant mice. J Endocrinol 2000; 164: 239–245.
Mayer-Kuckuk P, Gade TP, Buchanan IM, Doubrovin M, Ageyeva L, Bertino JR et al. High-resolution imaging of bone precursor cells within the intact bone marrow cavity of living mice. Mol Ther 2005; 12: 33–41.
Niemeyer M, Oostendorp RA, Kremer M, Hippauf S, Jacobs VR, Baurecht H et al. Non-invasive tracking of human haemopoietic CD34(+) stem cells in vivo in immunodeficient mice by using magnetic resonance imaging. Eur Radiol 2010; 20: 2184–2193.
Cunningham CH, Arai T, Yang PC, McConnell MV, Pauly JM, Conolly SM . Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles. Magn Reson Med 2005; 53: 999–1005.
Gilad AA, Walczak P, McMahon MT, Na HB, Lee JH, An K et al. MR tracking of transplanted cells with ‘positive contrast’ using manganese oxide nanoparticles. Magn Reson Med 2008; 60: 1–7.
Daldrup HE, Link TM, Blasius S, Strozyk A, Konemann S, Jurgens H et al. Monitoring radiation-induced changes in bone marrow histopathology with ultra-small superparamagnetic iron oxide (USPIO)-enhanced MRI. J Magn Reson Imaging 1999; 9: 643–652.
Acknowledgements
MRI was performed in the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility in the McKnight Brain Institute at the University of Florida, which is supported by the National High Field Magnet Laboratories. This work was supported by Grants RO1s HL7528 and HL70738 (NIH) to EWS and EEC-0506560 (NSF) to GAW and EWS. This work was supported by Grants RO1 HL75258 and HL70738 (NIH) to EWS and EEC-0506560 (NSF) to GAW and EWS. NEB performed or contributed to all experiments under advice from GAW and EWS. SK helped perform the SKL isolation, labeling and transplant experiments. LL helped perform the tibia bone window installations and acquired fluorescent videos of initial cell homing. All authors contributed equally in writing the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Bengtsson, N., Kim, S., Lin, L. et al. Ultra-high-field MRI real-time imaging of HSC engraftment of the bone marrow niche. Leukemia 25, 1223–1231 (2011). https://doi.org/10.1038/leu.2011.72
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2011.72
Keywords
This article is cited by
-
Extended time-lapse in vivo imaging of tibia bone marrow to visualize dynamic hematopoietic stem cell engraftment
Leukemia (2017)
-
Challenges, progress, and new directions in stem cell therapies: a new section launched inClinical and Translational Medicine
Clinical and Translational Medicine (2015)
-
New stem cell meeting on the Baltic Sea is launched
Leukemia (2012)
-
A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients
Leukemia (2012)
-
The bone marrow-expressed antimicrobial cationic peptide LL-37 enhances the responsiveness of hematopoietic stem progenitor cells to an SDF-1 gradient and accelerates their engraftment after transplantation
Leukemia (2012)