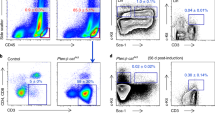
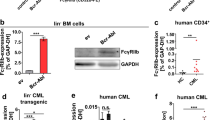
Abstract
Drug resistance is a growing concern with clinical use of tyrosine kinase inhibitors. Utilizing in vitro models of intrinsic drug resistance and stromal-mediated chemoresistance, as well as functional mouse models of progressive and residual disease, we attempted to develop a potential therapeutic approach designed to suppress leukemia recurrence following treatment with selective kinase inhibitors. The novel inhibitor of apoptosis (IAP), LCL161, was observed to potentiate the effects of tyrosine kinase inhibition against leukemic disease both in the absence and presence of a stromal protected environment. LCL161 enhanced the proapoptotic effects of nilotinib and PKC412, against leukemic disease in vitro and potentiated the activity of both kinase inhibitors against leukemic disease in vivo. In addition, LCL161 synergized in vivo with nilotinib to reduce leukemia burden significantly below the baseline level suppression exhibited by a moderate-to-high dose of nilotinib. Finally, LCL161 displayed antiproliferative effects against cells characterized by intrinsic resistance to tyrosine kinase inhibitors as a result of expression of point mutations in the protein targets of drug inhibition. These results support the idea of using IAP inhibitors in conjunction with targeted tyrosine kinase inhibition to override drug resistance and suppress or eradicate residual disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 1996; 2: 561–566.
Buchdunger E, Matter A, Druker BJ . Bcr-Abl inhibition as a modality of CML therapeutics. Biochim Biophys Acta 2001; 1551: M11–M18.
Deininger MW, Goldman JM, Melo JV . The molecular biology of chronic myeloid leukemia. Blood 2000; 96: 3343–3356.
Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001; 293: 876–880.
Weisberg E, Griffin JD . Mechanism of resistance to the ABL tyrosine kinase inhibitor STI571 in BCR/ABL-transformed hematopoietic cell lines. Blood 2000; 95: 3498–3505.
Weisberg E, Manley PW, Breitenstein W, Brüggen J, Cowan-Jacob SW, Ray A et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 2005; 7: 129–141.
Stirewalt DL, Radich JP . The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer 2003; 3: 650–665.
Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell 2002; 1: 433–443.
Stone RM, De Angelo J, Galinsky I, Estey E, Klimek V, Grandin W et al. PKC412 FLT3 inhibitor therapy in AML: results of a phase II trial. Ann Hematol 2004; 83 (Suppl 1): S89–S90.
Kumagai M, Manabe A, Pui CH, Behm FG, Raimondi SC, Hancock ML et al. Stroma-supported culture in childhood B-lineage acute lymphoblastic leukemia cells predicts treatment outcome. J Clin Invest 1996; 97: 755–760.
Du C, Fang M, Li Y, Li L, Wang X . Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000; 102: 33–42.
Liu Z, Sun C, Olejniczak ET, Meadows RP, Betz SF, Oost T et al. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature 2000; 408: 1004–1008.
Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X et al. Structural basis of IAP recognition by Smac/DIABLO. Nature 2000; 408: 1008–1012.
Galban S, Hwang C, Rumble JM, Oetjen KA, Wright CW, Boudreault A et al. Cytoprotective effects of IAPs revealed by a small molecule antagonist. Biochem J 2009; 417: 765–771.
Sensintaffar J, Scott FL, Peach R, Hager JH . XIAP is not required for human tumor cell survival in the absence of an exogenous death signal. BMC Cancer 2010; 10: 11.
Weisberg E, Kung AL, Wright RD, Moreno D, Catley L, Ray A et al. Potentiation of antileukemic therapies by Smac mimetic, LBW242: effects on mutant FLT3-expressing cells. Mol Cancer Ther 2007; 6: 1951–1961.
Daley GQ, Baltimore D . Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myeloid leukemia-specific p210 BCR-ABL protein. Proc Natl Acad Sci USA 1988; 85: 9312–9316.
Okuda K, Golub TR, Gilliland DG, Griffin JD . p210BCR-ABL, p190BCR-ABL, and TEL/ABL activate similar signal transduction pathways in hematopoietic cell lines. Oncogene 1996; 13: 1147–1152.
Sattler M, Salgia R, Okuda K, Uemura N, Durstin MA, Pisick E et al. The proto-oncogene product p120CBL and the adaptor proteins CRKL and c-CRK link c-ABL, p190BCR-ABL and p210BCR-ABL to the phosphatidylinositol-3′ kinase pathway. Oncogene 1996; 12: 839–846.
Matulonis U, Salgia R, Okuda K, Druker B, Griffin JD . IL-3 and p210 BCR-ABL activate both unique and overlapping pathways of signal transduction in a factor-dependent myeloid cell line. Exp Hematol 1993; 21: 1460–1466.
Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, Gilliland DG . FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood 2002; 99: 310–318.
Jiang J, Paez JG, Lee JC, Bo R, Stone RM, DeAngelo DJ et al. Identifying and characterizing a novel activating mutation of the FLT3 tyrosine kinase in AML. Blood 2004; 104: 1855–1858.
Cools J, Mentens N, Furet P, Fabbro D, Clark JJ, Griffin JD et al. Prediction of resistance to small molecule FLT3 inhibitors: implications for molecularly targeted therapy of acute leukemia. Cancer Res 2004; 64: 6385–6389.
Armstrong SA, Kung AL, Mabon ME, Silverman LB, Stam RW, Den Boer ML et al. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell 2003; 3: 173–183.
Chou T-C, Talalay P . Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enz Regul 1984; 22: 27–55.
Weisberg E, Wright RD, McMillin DW, Mitsiades C, Ray A, Barrett R et al. Stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-expressing leukemia cells. Mol Cancer Ther 2008; 7: 1121–1129.
Weisberg E, Barrett R, Liu Q, Stone R, Gray N, Griffin JD . FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat 2009; 12: 81–89.
Mony U, Jawad M, Seedhouse C, Russell N, Pallis M . Resistance to FLT3 inhibition in an in vitro model of primary AML cells with a stem cell phenotype in a defined microenvironment. Leukemia 2008; 22: 1395–1401.
Acknowledgements
JDG is supported by NIH Grant CA66996, and a Specialized Center of Research Award from the Leukemia and Lymphoma Society. JDG is also supported by NIH Grants CA36167 and DK50654.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JDG, RS, DF and ALK have a financial interest with Novartis Pharma AG. Employees of Novartis include DP, CS and LZ (formerly).
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Weisberg, E., Ray, A., Barrett, R. et al. Smac mimetics: implications for enhancement of targeted therapies in leukemia. Leukemia 24, 2100–2109 (2010). https://doi.org/10.1038/leu.2010.212
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2010.212
Keywords
This article is cited by
-
Increased migration and motility in XIAP-null cells mediated by the C-RAF protein kinase
Scientific Reports (2022)
-
BH3-mimetics: recent developments in cancer therapy
Journal of Experimental & Clinical Cancer Research (2021)
-
Characterization of p190-Bcr-Abl chronic myeloid leukemia reveals specific signaling pathways and therapeutic targets
Leukemia (2021)
-
TNF controls a speed-accuracy tradeoff in the cell death decision to restrict viral spread
Nature Communications (2021)
-
SMAC mimetics induce autophagy-dependent apoptosis of HIV-1-infected macrophages
Cell Death & Disease (2020)