Abstract
To determine whether the presence of Matrix metalloproteinases (MMPs) is associated with acute lung injury following cardiopulmonary bypass (CPB), we evaluated the activity and gene expression of matrix metalloproteinases-9 (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1) of lungs using rat model of CPB. Adult male Sprague-Dawley rats were randomly divided into three groups: Group I (underwent cannulation + heparinization only); group II (underwent 60 min of normothermic CPB); and group III (underwent 60 min of normothermic of CPB, which rats received doxycycline treat by filling stomach 1 week ahead of CPB). Lung injury was evaluated histologically. The enzyme activity of MMP-9 and TIMP-1 in the bronchoalveolar lavage fluid was detected by western-blot analysis. The expression of MMP-9 and TIMP-1 in lung tissue was assessed using reverse transcriptase–polymerase chain reaction method. We found there was significantly pulmonary edema and lung injury in groups II and III compared with group I, and the histological markers of pulmonary edema in the Group III were less pronounced in comparison with Group II. The MMP-9 activity and gene expression were increased significantly, but the TIMP-1 increased slowly in group II, and the ratio of MMP-9/TIMP-1 was imbalanced severely. More significantly, the MMP-9 decreased significantly and the TIMP-1 mRNA increased gradually in group III compared with group II, and the ratio of MMP-9/TIMP-1 was improved significantly. We concluded MMP-9 might have an important role in acute lung injury following CPB. TIMP-1 increased in the rats treated with doxycycline ahead and might compensate for the activity of MMP-9. The doxycycline might have the protective effect against acute lung injury following CPB.
Similar content being viewed by others
Main
Cardiac surgical procedures with cardiopulmonary bypass (CPB) are known to be associated with inflammatory response leading to several postoperative complications. Postoperative respiratory dysfunction is a well-recognized side effect of cardiac operations. About 25% of patients following open heart surgery are reported to have a significant respiratory impairment for at least 1 week after operation.1 However, the most significant proportion of this impairment is due to CPB, which usually leads to excessive interstitial pulmonary edema and subsequent abnormal gas exchange.2, 3
One essential step in the pathogenesis of respiratory impairment is the disruption of the pulmonary membrane and the infiltration of polymorphonuclear neutrophil granulocytes (PMNs) across the basement membrane into the pulmonary tissue. In this process, degradation of both basement membrane and extracellular matrix (ECM) is required. Matrix metalloproteinase-9 (MMP-9), a subgroup of zinc endopeptidases, has been identified to degrade basement membrane components including collagen type IV, fibronectin and gelatine.4 MMP-9 is therefore considered essential for PMN migration and alveolar capillary leakage and MMP-9 inhibition is a promising target for reduction or prevention of lung impairment. Increased MMP activity had been demonstrated in bronchoalveolar lavage fluid (BALF) following CPB in swine model.5
MMPs are inhibited by specific tissue inhibitors of metalloproteinases (TIMPs), which are ubiquitous natural inhibitors and form complexes with MMPs.6 Doxycycline is a tetracycline derivate, have nonspecific MMP inhibitory effects that are distinct from their antimicrobial action.7 Several studies in other pathological conditions have now demonstrated positive effects of MMP-9 inhibition by doxycycline on diseases that are accompanied by degradation of the basement membrane and ECM.8
We evaluated the enzyme activities of MMP-9 and TIMP-1 using western-blot analysis and their gene expressions using reverse transcriptase–polymerase chain reaction (RT-PCR) method in a rat model with CPB, which showed severe lung injury by histology.
MATERIALS AND METHODS
Animals and Groups
Male Sprague-Dawley rats (450–550 g) were used in the present study. All animals were housed in cages individually at standard conditions (12-hour light/dark cycle and 21 °C±1 °C). Animals received humane care in compliance with Principles of Laboratory Animal Care, formulated by the National Society for Medical Research, and the Guide for the Care and Use of Laboratory Animals, prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Pub. No. 85–23, revised 1996). The following experimental protocol was approved by local ethical committee.
Rats were randomized into three groups (n=12 for each group): Group I (control group, animals only cannulated for CPB but not underwent CPB); group II (CPB group, animals received 60 min of normothermic CPB procedure only); group III (CPB + Dox group, animals received doxycycline 30 mg/kg/day by orogastric tube for 7 days before 60 min of normothermic CPB procedure).
Rat Model of CPB
The rat CPB model was built as designed by Dong et al9 with some modifications. The methods for anesthesia, surgery, instrumentation of the heart, and physiological monitoring have been described previously. Rats were anesthetized with butaylone (60 mg/kg, intraperitoneal administration), and anesthesia was maintained with additional butaylone. The right femoral artery was cannulated for arterial pressure monitoring. Following administration of heparin (250 U/kg), a 16-gauge catheter, modified to a multiside-orifices cannula in the forepart, was inserted into the right atrium through the right jugular vein approach for vein drain line. A 22-gauge catheter for arterial infusion was introduced into tail artery. The mini-CPB circuit comprised a venous reservoir (10 ml), a specially designed membrane oxygenator (Micro-1, Kewei Medical Instrument, Guangzhou, China), a roller pump (BT00-300M, Lange, Shanghai, China). All components were connected with polyethylene tubing. Rectal temperature was monitored and kept at 37–38 °C by a heat lamp placed around the animal and the equipments. The circuit was primed with 10 ml of heparin 1 ml (250 U/kg), synthetic colloid 8 ml and sodium bicarbonate 1 ml. The flow rate was gradually added to 100 ml/kg/min and maintained for 60 min. Throughout the experiment, mean arterial pressure was maintained about 60 to 80 mm Hg. The right jugular vein was decannulated as soon as CPB was accomplished. According to the blood pressure, part of the remaining priming was infused through the tail artery. The tail and femoral artery catheters were removed and the incisions were sutured after procedure.
Specimen Collection
The rats were killed at the termination of CPB (T1), 6 h after termination of CPB (T2), respectively, in group II and III. In group I, rats were killed at the corresponding time points. Chest was opened and both lungs were removed intact and washed by PBS buffer when rats were killed. Right upper lung was served at −70 °C for RT-PCR. Left lungs were cannulated for bronchoalveolar lavage after weighted.
Lung Water
Left lungs were sharply dissected free of nonparenchymal tissue, with care taken to avoid contact with the tissue. Samples were placed in a dish and weighed. After bronchoalveolar lavage, the specimen was then oven-dried at 65 °C for 24 h and reweighed. Lung water was expressed as a ratio of wet to dry weight.
Bronchoalveolar Lavage
The left main bronchus was cannulated and secured so that the fluid didn't overflow. Ice saline (2 ml) was then injected as three aliquots of 6 ml each. Each aliquot was injected quickly and then withdrawn slowly three times to obtain an optimal BALF specimen. Combined aliquots of BALF were spun at 1000 g for 10 min to remove cells. Supernatant was frozen at −70 °C for subsequent chemical analysis and neutrophil was counted.
Western Blot Analysis for MMP-9 and TIMP-1 Activity in BALF
All rats BALF samples were electrophoresed on SDS-polyacrylamide gels (30 μg protein/sample was loaded for each immunoblot lane) according to a modified method by Laemmli10 and electroblotted onto Immobilon-P PVDF membranes. Membranes were blocked in Tris buffer (pH=7.4) containing 5% powdered milk for 1 h, washed and incubated with primary antibody of interest overnight at 4 °C. After incubation, samples were washed with borate saline (100 mM boric acid, 25 mM Na borate, 75 mM NaCl) and incubated with species-specific IgG horseradish peroxidase conjugates (at dilutions of 1:5000) for 1 h. Immunoblots were then developed using NEN chemiluminescent kits and the gel scanning imaging system.
RT-PCR for MMP-9 and TIMP-1 Gene Expression
Total cytoplasmic RNA was extracted from homogenized frozen lung tissue with RNA extraction solution (KGA1303, Nanjing KeyGen Biotech., China) according to the manufacturer's instruction and frozen at −70 °C until analysis. We used NCBI/ Primer-BLAST to design PCR primers from previously reported rat MMP-9, TIMP-1 and actin cDNA to arrange optimal annealing temperatures to the same degree. RT-PCR was performed with three-step cycling protocol using RT-PCR beads (Fermentas Maxima SYBR Green/ROX qPCR Master Mix, USA). We submitted 5 μg of RNA to the sterilized and no nuclease PCR tubes (0.2 ml) containing Oligo dT(18) (10 μM) 1.5 μl, nuclease-free double-distilled water (total volume 10 μl) at 70 °C for 10 min. Then, RNase inhibitor (40 U/μl) 0.5 μl, 10 × AMV Reaction Buffer 2.5 μl, dNTPs (10 mM) 1 μl, DTT (1 M) 1.5 μl, reverse transcriptase (AMV) 1 μl, and nuclease-free double-distilled water 8.5 μl were added into PCR tubes. Gently mixed the reactions without creating bubbles and centrifuged briefly. The mixed solution was incubated at 42 °C for 45 min, 72 °C for 10 min for reverse transcription. Gently mixed the reactions containing RT Mix (10 μL), template DNA (samples were diluted 10 times, 1 μl), forward primer (5 pmol/μl 1 μl), reverse primer (5 pmol/μl 1 μl), and nuclease-free double-distilled water 7 μl (total volume 20 μl). Placed the samples in the cycler and start the thermal cycling. We carried out 40 cycles of amplification, consisting of 95 °C for 25 s, 60 °C for 30 s, and 72 °C for 30 s on a thermal cycler (DA7600 Real-time Nucleic Acid Amplification Fluorescence Detection System, China), followed by a final extension at 72 °C for 10 min. After the PCR was completed, PCR products were analyzed by electrophoresis on a 2.0% agarose gel. Gene expression of rat MMP-9 and TIMP-1 was expressed in the relative density using the ratio of the band for the optical densities of rat MMP-9, and TIMP-1/rat b-actin.
Ultrastructure of Lung Tissue
Ultrastructure of lung tissue was observed by transmission electron microscopy (JEM-1200, Japan). Fresh lung specimens were fixed quickly in 2.5% cold glutaraldehyde solution for 2 h, in 1% osmium tetroxide for 1 h, then gradient dehydrated in acetone, embedded by Epon-812 resin, orientated by semi-thin section, sliced into 70 nm ultra-thin sections, and double electron stained by uranyl acetate–lead citrate, observed by transmission electron microscope.
Statistical Analysis
All values were expressed as mean±deviation. Data were analyzed using a commercially available statistics software package (SPSS for Windows v. 13.0, Chicago, IL, USA). We used one-way analysis of variance (ANOVA) with multiple comparisons for repeatedly measured parameters. Differences were considered significant at P<0.05.
RESULTS
Lung Water and Neutrophil Counts in BALF
The mean ratio of W/D and neutrophils counts in BALF in Groups I, II, and III were showed in Table 1. We noted significant differences between groups. At T2 time point, the W/D of group II was significantly higher than Groups I and III (8.9±0.9 vs 4.0±0.3, 5.6±0.8, P<0.01). At both two time points, the W/D of group III was lower than group II (4.5±0.7, 5.6±0.8 vs 6.7±0.8, 8.9±0.9, P<0.05), but higher than Group I, respectively, (4.5±0.7, 5.6±0.8 vs 3.6±0.4, 4.0±0.3, P<0.05). The change of neutrophils counts in BALF was similar with W/D.
Histology of Lung Injury Study
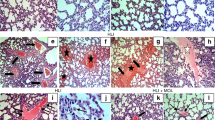
In group I, lungs showed normal pathological findings (Figure 1a). The alveolar epithelial walls were well maintained. Alveolus was lined fully with polygonal or cuboidal epithelial cells. There were abundant desmosomes between cells. Rich microvilli were observed at the alveolus cavity surface. Cytoplasm was rich in mitochondria and reticulum. In group II and III, the acute lung injury was marked by alveolar epithelial cell shedding, matrix exposure, vascular congestion, mitochondria of epithelial cells swelling obviously (Figures 1b and c), but the histological markers were less pronounced in III.
Pathological finding ( × 1200): (a) Group I, alveolar walls were well-maintained, abundant desmosomes and rich microvilli were observed at the alveolus cavity surface. (b) Group II, acute lung injury was observed. Alveolar epithelial cell shed, matrix exposure, vascular congestion and mitochondria of epithelial cells swell obviously. (c) Group III, acute lung injury was ameliorated by pretreatment with doxycycline. Fresh lung specimens were fixed quickly in 2.5% cold glutaraldehyde solution for 2 h, in 1% osmium tetroxide for 1 h, then gradient dehydrated in acetone, embedded by Epon-812 resin, orientated by semi-thin section, sliced into 70 nm ultra-thin sections, and double electron stained by uranyl acetate–lead citrate, observed by transmission electron microscope (JEM-1200, Japan). Group I (underwent cannulation + heparinization only); group II (underwent 60 min of normothermic cardiopulmonary bypass (CPB)); and group III (underwent 60 min of normothermic of CPB, which rats received doxycycline treat by filling stomach 1 week ahead of CPB).
The Activity of MMP-9 and TIMP-1 in BALF
The expression of activity of MMP-9 and TIMP-1 in the BALF by western-blot analysis was measured in all groups. In group II, activity of MMP-9 was significantly increased compared with control group (T1: 1.04±0.06 vs 1.19±0.09, P<0.01; 0.86±0.01 vs 1.14±0.04, P<0.01). In group III, the MMP-9 activity in BALF decreased significantly than group II at T2 time point (1.11±0.11 vs 0.86±0.01, P<0.01); but at T1 time point, there was no difference between them (1.09±0.04 vs 1.04±0.06, P=0.274). The change of TIMP-1 activity in BALF among three groups was similar with MMP-9. The activity of TIMP-1 in group II was higher significantly than group I (T1: 1.11±0.12 vs 1.15±0.08, P<0.01; T2: 1.01±0.09 vs 1.15±0.08, P<0.01), and lower significantly than group III (T1: 1.13±0.12 vs 1.11±0.12, P<0.05; T2: 0.98±0.02 vs 1.01±0.09, P<0.01; Table 2, Figure 2).
The activity of matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1) in bronchoalveolar lavage fluid (BALF) by western-blot analysis. Group I (underwent cannulation + heparinization only); group II (underwent 60 min of normothermic cardiopulmonary bypass (CPB)); and group III (underwent 60 min of normothermic of CPB, which rats received doxycycline treat by filling stomach 1 week ahead of CPB). T1, at the termination of CPB or operation; T2, 6 h after termination of CPB or operation.
The Expression of MMP-9 and TIMP-1 mRNA in Lung Tissue by RT-PCR
To see whether the observed changes in MMP activity reflect the level of mRNA for the enzyme we performed semiquantitative biplex RT-PCR assay, in which cDNA for b-actin, MMP-9 and TIMP-1 were simultaneously amplified. The levels of gene expression of rat MMP-9, and TIMP-1 were expressed as the ratio of the density of the band for the rat MMP-9, or TIMP-1 mRNA relative to that of rat b-actin.
The level of MMP-9 gene expression in Groups I, II and III were 0.96±0.23, 1.09±0.24, 4.90±0.90, 10.03±1.16, 3.51±0.49, and 8.87±1.20, respectively (Figure 3a). In group II, MMP-9 gene expression showed a dramatic increase at the late phase of termination of CPB compared with group I and III (P<0.001).
Matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression by using biplex reverse-transcription–polymerase chain reaction. The levels of gene expression of rat MMP-9, and TIMP-1 were expressed as the ratio of the density of the band for the rat MMP-9, or TIMP-1 mRNA relative to that of rat b-actin. (a) MMP-9 gene expression. In group II, MMP-9 gene expression was significantly higher than group I and III. (b) TIMP-1 gene expression. In group II and III, TIMP-1 gene expression showed a significant increase at the two time points, respectively, compared with group I. But there was no difference at the two time points, respectively, between group II and III. (c) Ratios of MMP-9 to TIMP-1 gene expression. In group II, the ratio was significantly higher than group I and III. Data are presented as the means±deviation. *P<0.01 as compared with group I and III. Group I (underwent cannulation + heparinization only); group II (underwent 60 min of normothermic cardiopulmonary bypass (CPB)); and group III (underwent 60 min of normothermic of CPB, which rats received doxycycline treat by filling stomach 1 week ahead of CPB). T1, at the termination of CPB or operation; T2, 6 h after termination of CPB or operation.
The level of TIMP-1 gene expression in Groups I, II and III were 0.91±0.20, 1.09±0.31, 3.08±0.63, 4.02±0.87, 3.11±0.91, and 4.62±0.85, respectively (Figure 3b). In group II and III, TIMP-1 gene expression showed a significant increase at the two time points, respectively, compared with group I (P<0.001), but there was no difference at the two time points, respectively, between group II and III (T1 P=0.957; T2 P=0.140).
The ratios of MMP-9 to TIMP-1 gene expression in three groups were 1.06±0.16, 1.07±0.25, 1.62±0.34, 2.59±0.66, 1.17±0.21 and 1.94±0.19, respectively (Figure 3c). In group II, the ratio was significantly higher than in the other groups (Group II vs Groups I, III: 1.62±0.34 vs 1.06±0.16, 1.17±0.21; 2.59±0.66 vs 1.07±0.25, 1.94±0.19, respectively, P=0.01, 0.03, 0.000, 0.003, respectively).
DISCUSSION
CPB has been shown to exert an inflammatory response within the lung, often resulting in postoperative pulmonary dysfunction that includes endothelial injury and increased microvascular permeability.11 The essential steps in the pathogenesis of lung injury depend on neutrophils and are mediated by the local release of oxygen radicals and proteases from PMNs.12 Activated neutrophils contribute to endothelial disruption, increased alveolar capillary permeability and pulmonary edema.13
The basement membrane is composed of the ECM molecules, which consist of a complex of type IV collagens. Degradation of ECM is controlled primarily by MMPs like MMP-9 (92 kDa, type IV collagenase).14
Low levels of MMPs are constitutively produced by stromal cells. Following injury or infection, proinflammatory cytokines (eg, interleukin (IL)-1 and tumor necrosis factor (TNF)-a ) stimulate an increase in stromal cell MMP production. During inflammation, alveolar macrophages and neutrophils constitute a significant additional source of MMPs in the lung. The major MMPs produced by alveolar macrophages are interstitial collagenase or MMP-1, the gelatinases A and B (MMP-2 and MMP-9), and a metalloelastase (MMP-12). MMP-9 is produced mainly by inflammatory cells, such as neutrophils, monocytes, macrophages, eosinophils, and lymphocytes.15
MMPs are inhibited by specific TIMPs, which are ubiquitous natural inhibitors and form complexes with MMPs.16 TIMP-1 is a main inhibitor for MMP-9. Evaluated TIMP-1 may be helpful to investigate the correlation between MMP-9 and pulmonary injury.
In the present study, we evaluated the activity and gene expression of MMP-9 and TIMP-1 in BALF and lung tissue in the rat model of CPB. In our model of rat lung injury following CPB, severe lung injury was apparent pathologically after CPB, concomitant with the appearance of interstitial edema and neutrophil infiltration. A large amount of MMP-9 was produced after the termination of CPB. Active MMP-9 enzyme and MMP-9 mRNA level increased significantly following CPB. Doxycycline therapy could significantly decrease MMP-9 activity in BALF compared with CPB group at T2 time point, and significantly decrease MMP-9 gene expression in lung tissue compared with CPB group at two time points, respectively. Several studies in other pathological conditions have now same demonstrated positive effects of MMP-9 inhibition by doxycycline on diseases that are accompanied by degradation of the basement membrane and ECM.8 Leukocytes, particularly macrophages, are major sources of MMP production in lung injury.17 We speculated that the initial increase of MMP-9 enzyme and MMP-9 mRNA were contributed by the leukocyte recruited to the pulmonary interstitial spaces.
TIMPs are produced to balance the action of MMPs.18 In our study, the activity of TIMP-1 in the BALF in group II increased compared with group I at 6 h after the termination of CPB, and decreased compared with group III. However, the change was minor compared with TIMP-1 mRNA levels. The TIMP-1 mRNA levels in lung tissue were significant after CPB. In group II and III, TIMP-1 gene expression showed a significant increase at the two time points, respectively, compared with group I. But the TIMP-1 mRNA growth rate and the rates were significantly lower than MMP-9 mRNA. So the ratio of MMP-9 mRNA to TIMP-1 mRNA level peaked after CPB in group II when lung injury was most severe. In group III, although MMP-9 gene expression increased compared with control group, the expression of MMP-9 was inhibited by doxycycline and the increase in TIMP-1 gene expression may have compensated for the MMP-9 activity and reduced the extent of lung injury. The ratio of MMP-9 mRNA to TIMP-1 mRNA level became minimal. It may be possible to propose this ratio as an early marker of lung injury.
This study has several limitations. A control group would allow assessment of the relative importance of CPB in lung injury and MMP production, because anesthesia and surgical interventions like the thoracotomy might at least partly contribute to these changes. A more detailed investigation, including more clinical and inflammatory parameters, especially markers of neutrophil and endothelial activation, would help to elucidate the mechanism of post-CPB lung injury more precisely.
In conclusion, we demonstrate increased MMP-9 activity and gene expression in the lung injury following CPB. Our data suggest that MMP-9 and the ratio of MMP-9 mRNA to TIMP-1 mRNA level may have an important role in lung injury following CPB. Our study does not exclude the role of other MMPs and proteases in lung injury. However, suppressing MMP-9 or increasing TIMP-1 may be a target for preventing or treating lung injury post-CPB.
References
Taggart DP, el-Fiky M, Carter R, et al. Respiratory dysfunction after uncomplicated cardiopulmonary bypass. Ann Thorac Surg 1993;56:1123–1128.
Speekenbrink R, van Oeveren W, Wildevuur C . Pathophysiology of cardiopulmonary bypass. In: Golstein D, Oz M (eds). Minimally Invasive Cardiac Surgery. 2nd edn. Humana Press: Totowa, NJ, 2004, pp. 3–18.
Asimakopoulos G, Smith PL, Ratnatunga CP, et al. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg 1999;68:1107–1115.
Martinez-Hernandez A, Amenta PS . The basement membrane in pathology. Lab Invest 1983;48:656–677.
Eichler W, Bechtel JF, Schumacher J, et al. A rise of MMP-2 and MMP-9 in bronchoalveolar lavage fluid is associated with acute lung injury after cardiopulmonary bypass in a swine model. Perfusion 2003;18:107–113.
Hijova E . Matrix metalloproteinases: their biological functions and clinical implications. Bratisl Lek Listy 2005;106:127–132.
Golub LM, Ramamurthy NS, McNamara TF, et al. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med 1991;2:297–321.
Lee CZ, Xu B, Hashimoto T, et al. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke 2004;35:1715–1719.
Dong GH, Xu B, Wang CT, et al. A rat model of cardiopulmonary bypass with excellent survival. J Surg Res 2005;123:171–175.
Laemmli UK . Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685.
Edmunds Jr LH . Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 1998;66:S12–S16.
Guice KS, Oldham KT, Caty MG, et al. Neutrophil-dependent, oxygen-radical mediated lung injury associated with acute pancreatitis. Ann Surg 1989;210:740–747.
Bhatia M, Saluja AK, Hofbauer B, et al. The effects of neutrophil depletion on a completely noninvasive model of acute pancreatitis-associated lung injury. Int J Pancreatol 1998;24:77–83.
Gipson TS, Bless NM, Shanley TP, et al. Regulatory effects of endogenous protease inhibitors in acute lung inflammatory injury. J Immunol 1999;162:3653–3662.
D'Ortho MP, Jarreau PH, Delacourt C, et al. Matrix metalloproteinase and elastase activities in LPS-induced acute lung injury in guinea pigs. Am J Physiol 1994;266:L209–L216.
Gomez DE, Alonso DF, Yoshiji H, et al. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol 1997;74:111–122.
Pardo A, Selman M, Ridge K, et al. Increased expression of gelatinases and collagenases in rat lungs exposed to 100% oxygen. Am J Respir Crit Care Med 1996;154:1067–1075.
Pardo A, Selman M, Ramírez R, et al. Production of collagenase and tissue inhibitor of metalloproteinases by fibroblasts derived from normal and fibrotic human lungs. Chest 1992;102:1085–1089.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
MMP-9 expression increases significantly in the lung after cardiopulmonary bypass (CPB), but TIMP-1 increases slowly and the MMP-9/TIMP-1 ratio is severely imbalanced. The ratio of MMP-9/TIMP-1 is improved by doxycycline. Suppressing MMP-9 or increasing TIMP-1 may be a target for preventing or treating lung injury post-CPB.
Rights and permissions
About this article
Cite this article
Wang, C., Li, D., Qian, Y. et al. Increased matrix metalloproteinase-9 activity and mRNA expression in lung injury following cardiopulmonary bypass. Lab Invest 92, 910–916 (2012). https://doi.org/10.1038/labinvest.2012.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2012.50