Abstract
Background
The study’s objective is to evaluate if Molsidomine (MOL), an anti-oxidant, anti-inflammatory, and anti-apoptotic drug, is effective in treating hyperoxic lung injury (HLI).
Methods
The study consisted of four groups of neonatal rats characterized as the Control, Control+MOL, HLI, HLI + MOL groups. Near the end of the study, the lung tissue of the rats were evaluated with respect to apoptosis, histopathological damage, anti-oxidant and oxidant capacity as well as degree of inflammation.
Results
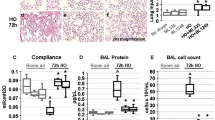
Compared to the HLI group, malondialdehyde and total oxidant status levels in lung tissue were notably reduced in the HLI + MOL group. Furthermore, mean superoxide dismutase, glutathione peroxidase, and glutathione activities/levels in lung tissue were significantly higher in the HLI + MOL group as compared to the HLI group. Tumor necrosis factor-α and interleukin-1β elevations associated with hyperoxia were significantly reduced following MOL treatment. Median histopathological damage and mean alveolar macrophage numbers were found to be higher in the HLI and HLI + MOL groups when compared to the Control and Control+MOL groups. Both values were increased in the HLI group when compared to the HLI + MOL group.
Conclusions
Our research is the first to demonstrate that bronchopulmonary dysplasia may be prevented through the protective characteristics of MOL, an anti-inflammatory, anti-oxidant, and anti-apoptotic drug.
Impact
-
Molsidomine prophylaxis significantly decreased the level of oxidative stress markers.
-
Molsidomine administration restored the activities of antioxidant enzymes.
-
Molsidomine prophylaxis significantly reduced the levels of inflammatory cytokines.
-
Molsidomine may provide a new and promising therapy for BPD in the future.
-
Molsidomine prophylaxis decreased lung damage and macrophage infiltration in the tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lignelli, E., Palumbo, F., Myti, D. & Morty, R. E. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 317, L832–L887 (2019).
Thomas, W. & Speer, C. P. Prevention and treatment of bronchopulmonary dysplasia: current status and future prospects. J. Perinatol. 27, S26–S32 (2007).
Hwang, J. S. & Rehan, V. K. Recent advances in bronchopulmonary dysplasia: pathophysiology, prevention, and treatment. Lung 196, 129–138 (2018).
Ozdemir, R. et al. Clarithromycin in preventing bronchopulmonary dysplasia in ureaplasma urealyticum-positive preterm infants. Pediatrics 128, e1496–e1501 (2011).
Bancalari, E. & Jain, D. Bronchopulmonary dysplasia: 50 years after the original description. Neonatology 115, 384–391 (2019).
Thebaud, B. et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Prim. 5, 78 (2019).
Tokuriki, S. et al. Treatment with geranylgeranylacetone induces heat shock protein 70 and attenuates neonatal hyperoxic lung injury in a model of bronchopulmonary dysplasia. Lung 195, 469–476 (2017).
Endesfelder, S., Strauss, E., Bendix, I., Schmitz, T. & Buhrer, C. Prevention of oxygen-induced inflammatory lung injury by caffeine in neonatal rats. Oxid. Med. Cell. Longev. 2020, 3840124 (2020).
Lingappan, K. et al. Beta-naphthoflavone treatment attenuates neonatal hyperoxic lung injury in wild type and Cyp1a2-knockout mice. Toxicol. Appl. Pharmacol. 339, 133–142 (2018).
Ofman, G. & Tipple, T. E. Antioxidants & bronchopulmonary dysplasia: beating the system or beating a dead horse? Free Radic. Biol. Med. 142, 138–145 (2019).
Cifci, A. et al. Ginger (Zingiber officinale) prevents severe damage to the lungs due to hyperoxia and inflammation. Turk. J. Med. Sci. 48, 892–900 (2018).
Savani, R. C. Modulators of inflammation in bronchopulmonary dysplasia. Semin. Perinatol. 42, 459–470 (2018).
Chen, S., Wu, Q., Zhong, D., Li, C. & Du, L. Caffeine prevents hyperoxia-induced lung injury in neonatal mice through Nlrp3 inflammasome and Nf-Kappab pathway. Respir. Res. 21, 140 (2020).
Morty, R. E. Recent advances in the pathogenesis of BPD. Semin. Perinatol. 42, 404–412 (2018).
Ehrhardt, H. et al. Absence of TNF-alpha enhances inflammatory response in the newborn lung undergoing mechanical ventilation. Am. J. Physiol. Lung Cell. Mol. Physiol. 310, L909–L918 (2016).
Li, H., Wang, G., Lin, S., Wang, C. & Zha, J. Loss of interleukin-6 enhances the inflammatory response associated with hyperoxia-induced lung injury in neonatal mice. Exp. Ther. Med. 17, 3101–3107 (2019).
Wang, S. H. & Tsao, P. N. Phenotypes of bronchopulmonary dysplasia. Int. J. Mol. Sci. 21, 6112 (2020).
Sherlock, L. G., Wright, C. J., Kinsella, J. P. & Delaney, C. Inhaled nitric oxide use in neonates: balancing what is evidence-based and what is physiologically sound. Nitric Oxide 95, 12–16 (2020).
Mandell, E. W., Kratimenos, P., Abman, S. H. & Steinhorn, R. H. Drugs for the prevention and treatment of bronchopulmonary dysplasia. Clin. Perinatol. 46, 291–310 (2019).
Akter, F., Coghlan, G. & de Mel, A. Nitric oxide in paediatric respiratory disorders: novel interventions to address associated vascular phenomena? Ther. Adv. Cardiovasc. Dis. 10, 256–270 (2016).
Kilic, T. et al. Protective and therapeutic effect of molsidomine on bleomycin-induced lung fibrosis in rats. Inflammation 37, 1167–1178 (2014).
Toplu, Y. et al. The protective role of molsidomine on the cisplatin-induced ototoxicity. Indian J. Otolaryngol. Head. Neck Surg. 66, 314–319 (2014).
Ozer, M. A. et al. Effects of molsidomine on retinopathy and oxidative stress induced by radiotheraphy in rat eyes. Curr. Eye Res. 42, 803–809 (2017).
Garcia-Cenador, M. B. et al. Comparative study of bacterial translocation control with nitric oxide donors and Cox2 inhibitor. Enferm. Infecc. Microbiol. Clin. 34, 490–498 (2016).
Bentli, R. et al. Molsidomine prevents cisplatin-induced hepatotoxicity. Arch. Med. Res. 44, 521–528 (2013).
Ozdemir, R. et al. Colchicine protects against hyperoxic lung injury in neonatal rats. Neonatology 102, 265–269 (2012).
Ozdemir, R. et al. Dexpanthenol therapy reduces lung damage in a hyperoxic lung injury in neonatal rats. J. Matern. Fetal Neonatal Med. 29, 1801–1807 (2016).
Gurpinar, T. et al. The effects of the melatonin treatment on the oxidative stress and apoptosis in diabetic eye and brain. Sci. World J. 2012, 498489 (2012).
Kamisli, S., Ciftci, O., Taslidere, A., Turkmen, N. B. & Ozcan, C. The beneficial effects of 18 beta-glycyrrhetinic acid on the experimental autoimmune encephalomyelitis (EAE) in C57bl/6 mouse model. Immunopharmacol. Immunotoxicol. 40, 344–352 (2018).
Alvira, C. M. & Morty, R. E. Can we understand the pathobiology of bronchopulmonary dysplasia? J. Pediatr. 190, 27–37 (2017).
Berkelhamer, S. K. & Farrow, K. N. Developmental regulation of antioxidant enzymes and their impact on neonatal lung disease. Antioxid. Redox Signal. 21, 1837–1848 (2014).
Antosova, M. et al. Physiology of nitric oxide in the respiratory system. Physiol. Res. 66, S159–S172 (2017).
Wink, D. A. et al. Nitric oxide protects against the cytotoxic effects of reactive oxygen species. Ann. NY Acad. Sci. 738, 265–278 (1994).
Chander, V. & Chopra, K. Renal protective effect of molsidomine and L-arginine in ischemia-reperfusion induced injury in rats. J. Surg. Res. 128, 132–139 (2005).
Witkowski, S. M. et al. Analysis of interleukins 6, 8, 10 and 17 in the lungs of premature neonates with bronchopulmonary dysplasia. Cytokine 131, 155118 (2020).
Davies, P. L. et al. Relationship of proteinases and proteinase inhibitors with microbial presence in chronic lung disease of prematurity. Thorax 65, 246–251 (2010).
Rodriguez-Pena, A., Garcia-Criado, F. J., Eleno, N., Arevalo, M. & Lopez-Novoa, J. M. Intrarenal administration of molsidomine, a molecule releasing nitric oxide, reduces renal ischemia-reperfusion injury in rats. Am. J. Transplant. 4, 1605–1613 (2004).
Kang, J. L. et al. Inhaled nitric oxide attenuates acute lung injury via inhibition of nuclear factor-kappa B and inflammation. J. Appl. Physiol. 92, 795–801 (2002).
Kinsella, J. P. et al. Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease. Pediatr. Res. 41, 457–463 (1997).
Xia, H. P., Huang, G. Y., Zhu, J. X. & Sun, B. Effect of inhalation of nebulized no donor substance on acute hypoxic lung injury in newborn piglets. Chin. Med. J. 121, 1622–1626 (2008).
Thomassen, M. J. & Kavuru, M. S. Human alveolar macrophages and monocytes as a source and target for nitric oxide. Int. Immunopharmacol. 1, 1479–1490 (2001).
Cao, L. et al. Regulation of activity of nuclear factor-kappaB and activator protein-1 by nitric oxide, surfactant and glucocorticoids in alveolar macrophages from piglets with acute lung injury. Acta Pharmacol. Sin. 24, 1316–1323 (2003).
Zhang, D. et al. Autophagy inducers restore impaired autophagy, reduce apoptosis, and attenuate blunted alveolarization in hyperoxia-exposed newborn rats. Pediatr. Pulmonol. 53, 1053–1066 (2018).
May, M. et al. Apoptosis and proliferation in lungs of ventilated and oxygen-treated preterm infants. Eur. Respir. J. 23, 113–121 (2004).
Tang, J. R. et al. Early inhaled nitric oxide treatment decreases apoptosis of endothelial cells in neonatal rat lungs after vascular endothelial growth factor inhibition. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L1271–L1280 (2007).
Li, C. Q. & Wogan, G. N. Nitric oxide as a modulator of apoptosis. Cancer Lett. 226, 1–15 (2005).
Wright, C. J. et al. No inhibits hyperoxia-induced NF-KappaB activation in neonatal pulmonary microvascular endothelial cells. Pediatr. Res. 68, 484–489 (2010).
DeMeester, S. L. et al. Nitric oxide inhibits stress-induced endothelial cell apoptosis. Crit. Care Med. 26, 1500–1509 (1998).
Jobe, A. H. Mechanisms of lung injury and bronchopulmonary dysplasia. Am. J. Perinatol. 33, 1076–1078 (2016).
Bonadies, L. et al. Present and future of bronchopulmonary dysplasia. J. Clin. Med. 9, 1539 (2020).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
R.O. and I.K.G. participated in the study design, analyzed data, performed experiments, interpreted results, and edited the manuscript. S.T. and A.C.T. participated in the study design, prepared figures, interpreted results, analyzed data, performed experiments, and edited the manuscript. K.T. and C.C.G. contributed to conceptualizing the study, interpreted results, performed experiments, and prepared figures. H.T., M.F.D., H.K., and M.A. conceptualized the study and its design, interpreted results, performed experiments and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All procedures performed in studies involving animals were in accordance with the ethical standards of the Ethical Committee of Experimental Animals of the Faculty of Medicine in Inonu University, at which the studies were conducted.
Consent for publication
The manuscript is approved by all authors for publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aslan, M., Gokce, I.K., Turgut, H. et al. Molsidomine decreases hyperoxia-induced lung injury in neonatal rats. Pediatr Res 94, 1341–1348 (2023). https://doi.org/10.1038/s41390-023-02643-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02643-w