Abstract
Objective:
Oral colostrum priming (OCP) after birth in preterm infants is associated with improved weight gain and modification of the oral immunomicrobial environment. We hypothesized that OCP would modify salivary immune peptides and the oral microbiota in preterm infants.
Study design:
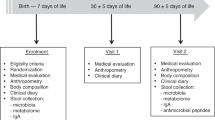
We conducted a prospective, randomized clinical trial to determine the effects of OCP on salivary immune peptide representation in preterm infants (<32 weeks completed gestation at birth). Saliva samples were collected before and after OCP. Salivary immune peptide representation was determined via mass spectroscopy. Oral microbiota representation was determined via sequencing of the 16S rRNA gene.
Results:
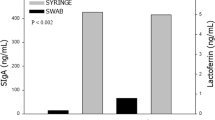
Neonates who received OCP (n=48) had a 16-day reduction in the median length of hospitalization as compared with infants who did not receive OCP (n=51). No differences in salivary immune peptide sequence representation before OCP between groups were found. Longitudinal changes in peptides were detected (lysozyme C, immunoglobulin A, lactoferrin) but were limited to a single peptide difference (α-defensin 1) between primed and unprimed infants after OCP. We found no difference in microbial diversity between treatment groups at any time point, but diversity decreased significantly over time in both groups. OCP treatment marginally modified oral taxa with a decline in abundance of Streptococci in the OCP group at 30 days of life.
Conclusions:
OCP had neither an effect on the salivary peptides we examined nor on overall oral bacterial diversity and composition. Infants who received OCP had a reduced length of hospitalization and warrants further investigation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF . Role of human milk in extremely low birth weight infants' risk of necrotizing enterocolitis or death. J Perinatol 2009; 29 (1): 57–62.
Hylander MA, Strobino DM, Dhanireddy R . Human milk feedings and infection among very low birth weight infants. Pediatrics 1998; 102 (3): E38.
Civardi E, Garofoli F, Mazzucchelli I, Angelini M, Manzoni P, Stronati M . Enteral nutrition and infections: the role of human milk. Early Hum Dev 2014; 90 (Suppl 1): S57–S59.
Jakaitis BM, Denning PW . Human breast milk and the gastrointestinal innate immune system. Clin Perinatol 2014; 41 (2): 423–435.
Al-Shehri SS, Knox CL, Liley HG, Cowley DM, Wright JR, Henman MG et al. Breastmilk–saliva interactions boost innate immunity by regulating the oral microbiome in early infancy. PLoS One 2015; 10 (9): e0135047.
Holgerson PL, Vestman NR, Claesson R, Ohman C, Domellof M, Tanner AC et al. Oral microbial profile discriminates breast-fed from formula-fed infants. J Pediatr Gastroenterol Nutr 2013; 56 (2): 127–136.
Biesbroek G, Bosch AA, Wang X, Keijser BJ, Veenhoven RH, Sanders EA et al. The impact of breastfeeding on nasopharyngeal microbial communities in infants. Am J Respir Crit Care Med 2014; 190 (3): 298–308.
Maron JL, Hwang JS, Pathak S, Ruthazer R, Russell RL, Alterovitz G . Computational gene expression modeling identifies salivary biomarker analysis that predict oral feeding readiness in the newborn. J Pediatr 2015; 166 (2): 282–288.e285.
Rodriguez NA, Meier PP, Groer MW, Zeller JM, Engstrom JL, Fogg L . A pilot study to determine the safety and feasibility of oropharyngeal administration of own mother's colostrum to extremely low-birth-weight infants. Adv Neonatal Care 2010; 10 (4): 206–212.
Sohn K, Kalanetra KM, Mills DA, Underwood MA . Buccal administration of human colostrum: impact on the oral microbiota of premature infants. J Perinatol 2016; 36 (2): 106–111.
Lee J, Kim HS, Jung YH, Choi KY, Shin SH, Kim EK et al. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics 2015; 135 (2): e357–e366.
Seigel JK, Smith PB, Ashley PL, Cotten CM, Herbert CC, King BA et al. Early administration of oropharyngeal colostrum to extremely low birth weight infants. Breastfeed Med 2013; 8 (6): 491–495.
Romano-Keeler J, Moore DJ, Wang C, Brucker RM, Fonnesbeck C, Slaughter JC et al. Early life establishment of site-specific microbial communities in the gut. Gut microbes 2014; 5 (2): 192–201.
Edwards U, Rogall T, Blocker H, Emde M, Bottger EC . Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res 1989; 17 (19): 7843–7853.
Fierer N, Hamady M, Lauber CL, Knight R . The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci USA 2008; 105 (46): 17994–17999.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010; 7 (5): 335–336.
Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res 2011; 21 (3): 494–504.
Lozupone C, Knight R . UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 2005; 71 (12): 8228–8235.
Lozupone C, Hamady M, Knight R . UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinform 2006; 7: 371.
Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH et al. The human oral microbiome. J Bacteriol 2010; 192 (19): 5002–5017.
Manktelow B, Draper ES, Field C, Field D . Estimates of length of neonatal stay for very premature babies in the UK. Arch Dis Child Fetal Neonatal Ed 2010; 95 (4): F288–F292.
Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006; 118 (2): 511–521.
Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS . Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol 2003; 30 (7): 644–654.
Denepitiya L, Kleinberg I . A comparison of the microbial compositions of pooled human dental plaque and salivary sediment. Arch Oral Biol 1982; 27 (9): 739–745.
Sakamoto M, Takeuchi Y, Umeda M, Ishikawa I, Benno Y . Application of terminal RFLP analysis to characterize oral bacterial flora in saliva of healthy subjects and patients with periodontitis. J Med Microbiol 2003; 52 (Pt 1): 79–89.
Tanaka S, Kobayashi T, Songjinda P, Tateyama A, Tsubouchi M, Kiyohara C et al. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Microbiol 2009; 56 (1): 80–87.
Mazmanian SK, Round JL, Kasper DL . A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008; 453 (7195): 620–625.
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013; 155 (7): 1451–1463.
De La Cochetiere MF, Durand T, Lepage P, Bourreille A, Galmiche JP, Dore J . Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. J Clin Microbiol 2005; 43 (11): 5588–5592.
Jernberg C, Lofmark S, Edlund C, Jansson JK . Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J 2007; 1 (1): 56–66.
Gronlund MM, Lehtonen OP, Eerola E, Kero P . Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr 1999; 28 (1): 19–25.
Hendricks-Munoz KD, Xu J, Parikh HI, Xu P, Fettweis JM, Kim Y et al. Skin-to-skin care and the development of the preterm infant oral microbiome. Am J Perinatol 2015; 32 (13): 1205–1216.
Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med 2016; 22: 250–253.
Sampaio-Maia B, Monteiro-Silva F . Acquisition and maturation of oral microbiome throughout childhood: an update. Dent Res J (Isfahan) 2014; 11 (3): 291–301.
Cacho N, Neu J . Manipulation of the intestinal microbiome in newborn infants. Adv Nutr 2014; 5 (1): 114–118.
Acknowledgements
We gratefully acknowledge the families who allowed their infants to participate in the study. We also acknowledge Dr Judy Aschner for her assistance with the study design, as well as Theresa Rogers and Amy Beller for their assistance with patient enrollment and sample collection. This support for this work was provided by the Thrasher Fund and NIH/NIGMS (GM106143 (to JLW)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Perinatology website
Supplementary information
Rights and permissions
About this article
Cite this article
Romano-Keeler, J., Azcarate-Peril, M., Weitkamp, JH. et al. Oral colostrum priming shortens hospitalization without changing the immunomicrobial milieu. J Perinatol 37, 36–41 (2017). https://doi.org/10.1038/jp.2016.161
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.161
This article is cited by
-
Oropharyngeal administration of colostrum targeting gut microbiota and metabolites in very preterm infants: protocol for a multicenter randomized controlled trial
BMC Pediatrics (2023)
-
A randomized controlled trial of oropharyngeal therapy with mother’s own milk for premature infants
Journal of Perinatology (2023)
-
Oropharyngeal colostrum therapy reduces the incidence of ventilator-associated pneumonia in very low birth weight infants: a systematic review and meta-analysis
Pediatric Research (2021)
-
Colostrum oropharyngeal immunotherapy for very low birth weight preterm infants: protocol of an intervention study
BMC Pediatrics (2020)
-
Effects of oropharyngeal administration of colostrum on the incidence of necrotizing enterocolitis, late-onset sepsis, and death in preterm infants: a meta-analysis of RCTs
European Journal of Clinical Nutrition (2020)