Abstract
Background
Early administration of colostrum may provide preterm infants with immune components. Previous studies illustrating the effects of oral colostrum (OC) have been confounded by the coincidence of enteral feedings.
Objective
To quantify OC absorption, as measured by urinary sIgA and lactoferrin, in preterm infants prior to enteral feedings.
Materials and methods
Colostrum was obtained from mothers delivering infants ≤32 weeks and ≤1500 g. sIgA and lactoferrin were measured in infant urine, and microflora in saliva and tracheal aspirates were characterized.
Results
Urinary sIgA and lactoferrin were significantly greater in infants receiving OC by syringe compared to swab (p < 0.002). Urinary sIgA correlated with the total number of doses in 72 h (R2 = 43%, p < 0.01).
Conclusions
Administration of OC by syringe and higher cumulative dose are associated with increased absorption of sIgA and lactoferrin, and early dosing may contribute to a more diverse tracheal microbiome.
Similar content being viewed by others
Introduction
Colostrum from mothers of preterm infants contains high concentrations of immune factors [1,2,3]. Early exposure to colostrum, however, is limited not only due to maternal reasons but because preterm infants often have delayed feedings and small quantities of milk may be difficult to administer with intragastric tube feedings [4,5,6]. Application of colostrum to the buccal mucosa (oral colostrum, OC) may provide early immunologic benefit to the preterm infant and provision of colostrum may facilitate maternal milk production [7].
Although a previous investigation reported absorption of human milk proteins, sIgA and lactoferrin, as a function of OC exposure, coincident intragastric tube feedings of human milk may have contributed to the outcomes [8]. Additional investigations suggested that human milk as the first feeding and the feeding at discharge were more likely after receipt of OC [8, 9]. OC also may impact the oral microbiome by stimulating lymphoid tissue and modulating bacterial adhesion [10, 11]. It has been suggested that OC alters bacterial colonization in the trachea of intubated infants and decreases ventilator-associated airway infections [10].
Despite the apparent safety and feasibility of OC exposure, there are no standards for the collection of milk, transfer of milk to the infant, and frequency and method of administration [6, 9, 10]. The aims of this pilot study were to document the use of OC in a preterm infant population, determine whether selected human milk immune proteins are absorbed following OC exposure in absence of feeding, and to characterize the effects of OC on the oral and tracheal microflora.
Materials and methods
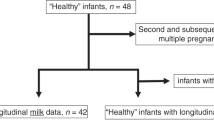
Preterm infants (≤32 weeks gestation and ≤1500 g birth weight) who did not receive enteral feedings for 72 h were eligible for recruitment and enrolled from February 2016 to December 2017. Study personnel reviewed the timing and dosing of OC prior to 72 h, collected urine samples at 72 h, and, if infants were intubated collected samples of tracheal secretions at 72 h and 7 days. The study was approved by the Northwell Health Institutional Review Board, and informed consent was obtained from parents. Infants with major congenital abnormalities or those expected not to survive >1 week were not enrolled. Maternal decision for breastfeeding was not a requirement for enrollment, but 90% of mothers eventually expressed their milk.
OC administration
The routine care of infants in the neonatal intensive care unit (NICU) included lactation consultants working with mothers to ensure early milk expression and provision of OC to infants as soon as it is available, with no specific contraindications to its use. Labor & Delivery personnel were asked to discuss manual milk expression with mothers. NICU staff were instructed on administration of OC to the buccal mucosa bilaterally every 3 h as available. Initially, cotton swabs were used, but in October 2016 this practice was changed to the use of tuberculin syringes to deliver 0.1 mL to each buccal surface. The number and frequency of doses were counted.
Determination of urinary secretory IgA and lactoferrin
Urine was collected at 72 h after birth, before any enteral feedings were initiated, using a sterile collection bag in the diaper. Samples were aliquoted and stored at −80 °C until analyzed. Secretory IgA (ABCAM, Cambridge, MA) and lactoferrin (Eagle Biosciences, Nashua, NH) were analyzed by enzyme-linked immunosorbent assay. Urinary creatinine (Cayman Chemical, Ann Arbor, Michigan) was used to adjust for differences in urinary output.
Microbiome analysis
Samples of saliva were collected at 72 h. Tracheal secretions were obtained via in-line suctioning at 72 h and day 7 if infants were intubated. DNA was isolated from saliva and tracheal samples using a ZymoBiomics DNA Mini Kit (Irvine, CA). Polymerase chain reaction (PCR) of the 16 s ribosomal DNA (16 s rDNA) was performed according to the methods of Tannock [12]. The PCR product was sequenced using the Illumina-curated version of the May 2013 release of the Greengene Consortium Database. Shannon Diversity Index (SDI) was calculated as a measure of genus diversity [13].
Demographic and outcome data
The following data were obtained from the electronic medical record: timing and frequency of OC administration in the first 72 h; birth weight; gestational age; receipt of antibiotics or other medications; comorbidities including infection, necrotizing enterocolitis, bronchopulmonary dysplasia, retinopathy of prematurity; and timing and type of enteral nutrition. Maternal demographics, including receipt of antibiotics and/or chorioamnionitis, were recorded.
Statistics
As a pilot study, no power analysis or calculation of sample size was performed. Univariate and multivariable regression were used to determine relationships between OC dose (frequency) and urinary concentrations of sIgA and lactoferrin. Univariable linear regression analyses were performed to examine the association between SDI and timing of initial OC and cumulative number of OC administrations. Analysis was conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
The time to receipt of first OC was 32 ± 22 h (mean ± SD, range 4–66 h). OC was given to 15 (31%) and 20 (42%) infants by 24 and 36 h, respectively. During the 72 h study interval, infants received a median of 7.5 doses (range 1–16). The number of OC doses received in the first 24 h was predictive of the total OC doses in 72 h, R2 = 32%, p < 0.001. Of 48 consecutive infants enrolled, 16 (33%) did not receive OC by 72 h. When comparing OC and no OC infants, there were no differences in birth weight (overall mean 895 ± 266 g), gestational age (overall mean 26.6 ± 2.5 weeks), and incidence of Cesarean delivery (overall 81%).
OC was administered to the buccal mucosa by swab in 20 (42%) infants and by syringe in 28 (58%) infants. There were no differences between swab vs syringe administration for birth weight, gestational age, and number of colostrum doses. The method of OC administration was not associated with the number of OC doses by 72 h or the time to full feedings (25 ± 15 days).
Colostrum administration by syringe was associated with significantly greater urinary sIgA and lactoferrin concentrations than by swab (median 427 vs 4 ng/mL and 5 vs 0.8 ng/mL, respectively, Fig. 1). These findings were not affected by normalizing to creatinine as a marker of urine concentration. There was a direct relationship between urinary sIgA and the number of OC doses by 72 h, R2 = 43%, p = 0.001, Fig. 2.
In this pilot study, we found no significant differences between cumulative doses of OC and distribution of bacterial genera, or bacterial diversity as quantified by SDI, in saliva at 72 h. Similarly, there was no significant association between the time of first OC dose and the composition or SDI of the oral or tracheal microbiota at 72 h. There was a trend toward decreased SDI in trachea on day 7 with increased time to first OC administration, R2 = 19%, p = 0.088. Method of OC administration was not associated with the incidence of sepsis (34%), bronchopulmonary dysplasia (38%), and necrotizing enterocolitis (6%), but differences were observed between swab and syringe administration for retinopathy of prematurity, 26% vs 0%, p = 0.016. Overall, infants with sepsis had less lactoferrin absorbed than those without sepsis, 1.2 ± 1.4 vs 4.3 ± 3.7 ng/mL, p = 0.025.
Discussion
Although OC is emerging as a part of routine care for preterm infants, practices differ widely. Using urine concentrations as a marker for intestinal absorption of milk-derived immune proteins, we found that uptake of sIgA and lactoferrin were optimal when delivered to the buccal mucosa via tuberculin syringe rather than by cotton swab [14]. Of note, urinary sIgA was positively associated with total number of OC doses received. This finding is novel because all sampling was done prior to the initiation of enteral feedings, so absorbed sIgA and lactoferrin are attributable solely to intake via OC. Early and ongoing administration of OC by syringe is likely to optimize innate immune defenses in preterm infants exposed to a human milk diet.
It was concerning that, despite the emphasis on the importance of human milk and OC in our NICU, the median time to first OC administration was more than 24 h after delivery and less than half of the infants received OC in the first 36 h. Since early OC results in greater cumulative doses administered and, therefore, greater immune protein concentrations, it is necessary to further emphasize the importance of early milk expression to all staff. These findings support the idea that mothers of preterm infants face unique challenges to early milk production, likely related either to insufficient breast priming, stress, or medical diagnoses that contributed to preterm labor. Although there was staff education, more direct support from staff in Labor & Delivery and NICU is required. We now emphasize the importance of immediate postpartum milk expression during antenatal consultations for all mothers likely to deliver prematurely. We have empowered our staff to encourage transfer of any amount of milk to the NICU immediately after delivery.
OC did not significantly alter the composition of the oral and tracheal microflora. The trend toward decreased microbial diversity in the trachea with increased time to first OC suggests that early OC may contribute to development of a more robust and protective microbiome. The microbiome data may contribute to understanding the association between a human milk diet in preterm infants and protection from bronchopulmonary dysplasia [15].
Limitations of this study include the lack of randomization. The change from swab to syringe OC was adopted throughout the NICU and observations were not concurrent, so they may be confounded by other alterations in practice. As all recruitment was done within a 2 year period, this effect is unlikely to be significant. The differences in retinopathy of prematurity, however, reflect the practice changes in monitoring oxygen satuartions during this time period. This study enrolled infants who were not ordered to receive enteral feedings until after 72 h. As this practice is contrary to our NICU guidelines of early enteral nutrition, it is likely that study infants were sicker than the usual preterm population, and provides a rationale for the observations of delays in time to full enteral feeding and the higher incidence of comorbidities.
In summary, we found that immune proteins in human milk are absorbed after early OC administration and that early colostrum is best administered onto the buccal mucosa by syringe. Greater cumulative OC doses are associated with increased absorption of sIgA and lactoferrin. The delays in receipt of OC support the need for further efforts to obtain milk as soon as possible after preterm delivery.
References
Dvorak B, Fituch CC, Williams CS, Hurst NM, Schanler RJ. Increased epidermal growth factor levels in human milk of mothers with extremely premature infants. Pediatr Res. 2003;54:15–9.
Trend S, Strunk T, Lloyd ML, Kok CH, Metcalfe J, Geddes DT, et al. Levels of innate immune factors in preterm and term mothers’ breast milk during the 1st month postpartum. Br J Nutr. 2016;115:1178–93.
Koenig A, Diniz EMA, Barbarosa SFC, Vaz FAC. Immunologic factors in human milk: the effects of gestational age and pasteurization. J Hum Lact. 2005;21:439–43.
Meier PP, Engstrom JL, Patel AL, Jegier BJ, Bruns NE. Improving the use of human milk during and after the NICU stay. Clin Perinatol. 2010;37:217–45.
Nasuf AWA, Ojha S, Dorling J. Oropharyngeal colostrum in preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2018;9:CD011921.
Pletsch D, Ulrich C, Angelini M, Fernandes G, Lee DS. Mothers’ “liquid gold”: a quality improvement initiative to support early colostrum delivery via oral immune therapy (OIT) to premature and critically ill newborns. Nurs Leadersh. 2013;26:34–42.
Parker LA, Sullivan S, Krueger C, Kelechi T, Mueller M. Effect of early breast milk expression on milk volume and timing of lactogenesis stage II among mothers of very low birth weight infants: a pilot study. J Perinatol. 2012;32:1–5.
Lee J, Kim HS, Jung YH, Choi KY, Shin SH, Kim EK, et al. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics. 2015;135:e357–66.
Snyder R, Herdt A, Mejias-Cepeda N, Ladino J, Crowley K, Levy P. Early provision of oropharyngeal colostrum leads to sustained breast milk feedings in preterm infants. Pediatr Neonatol. 2017;58:534–40.
Rodriguez NA, Meier PP, Groer MW, Zeller JM, Engstrom JL, Fogg L. A pilot study to determine the safety and feasibility of oropharyngeal administration of own mother’s colostrum to extremely low-birth-weight infants. Adv Neonatal Care. 2010;10:206–12.
Sohn K, Kalanetra KM, Underwood MA. Buccal administration of human colostrum: impact on the oral microbiota of premature infants. J Perinatol. 2016;36:106–11.
Tannock GW. Identification of lactobacilli and bifidobacteria. Curr Issues Mol Biol. 1999;1:53–64.
Tuomisto H. A consistent terminology for quantifying species diversity? Yes, it does exist. Oecologia. 2010;164:853–60.
Schanler RJ, Goldblum RM, Garza C, Goldman AS. Enhanced fecal excretion of selected immune factors in very low birth weight infants fed fortified human milk. Pediatr Res. 1986;20:711–5.
Spiegler J, Preuß M, Gebauer C, Bendiks M, Herting E, Göpel W, German Neonatal Network (GNN). Does breastmilk influence the development of bronchopulmonary dysplasia? J Pediatr. 2016;169:76–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maffei, D., Brewer, M., Codipilly, C. et al. Early oral colostrum administration in preterm infants. J Perinatol 40, 284–287 (2020). https://doi.org/10.1038/s41372-019-0556-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0556-x
This article is cited by
-
The effect of oropharyngeal mother’s milk on nutritional outcomes in preterm infants: a randomized controlled trial
BMC Pediatrics (2024)
-
Oropharyngeal administration of colostrum targeting gut microbiota and metabolites in very preterm infants: protocol for a multicenter randomized controlled trial
BMC Pediatrics (2023)
-
A randomized controlled trial of oropharyngeal therapy with mother’s own milk for premature infants
Journal of Perinatology (2023)
-
Liquid gold: do we need to fraction fresh colostrum for oral immunotherapy in premature infants?
International Breastfeeding Journal (2022)
-
Increasing early exposure to mother’s own milk in premature newborns
Journal of Perinatology (2022)