Abstract
Objective:
We examined the effects of an aerobic exercise intervention on adiposity outcomes that may be involved in the association between physical activity and breast cancer risk.
Design:
This study was a two-centre, two-armed, randomized controlled trial. The 1-year-long exercise intervention included 45 min of moderate-to-vigorous aerobic exercise five times per week, with at least three of the sessions being facility based. The control group was asked not to change their activity and both groups were asked not to change their diet.
Subjects:
A total of 320 postmenopausal, sedentary, normal weight-to-obese women aged 50–74 years who were cancer-free, nondiabetic and nonhormone replacement therapy users were included in this study.
Measurements:
Anthropometric measurements of height, weight and waist and hip circumferences; dual energy X-ray absorptiometry measurements of total body fat; and computerized tomography measurements of abdominal adiposity were carried out.
Results:
Women in the exercise group exercised a mean of 3.6 days (s.d.=1.3) per week and 178.5 min (s.d.=76.1) per week. Changes in all measures of adiposity favored exercisers relative to controls (P<0.001). The mean difference between groups was: −1.8 kg for body weight; −2.0 kg for total body fat; −14.9 cm2 for intra-abdominal fat area; and −24.1 cm2 for subcutaneous abdominal fat area. A linear trend of greater body fat loss with increasing volume of exercise was also observed.
Conclusion:
A 1-year aerobic exercise program consistent with current public health guidelines resulted in reduced adiposity levels in previously sedentary postmenopausal women at higher risk of breast cancer.
Similar content being viewed by others
Introduction
Increased adiposity negatively impacts health including overall mortality; a wide range of chronic diseases including diabetes, coronary heart disease and cancer; and a poor quality of life.1, 2, 3, 4 Overweight and obesity are prevalent worldwide,5, 6, 7 exceeding 60% in the United States,7 and globally their rates are increasing.8, 9, 10, 11, 12 Thus, developing and testing interventions to treat this condition is a priority, particularly in the context of effecting downstream chronic diseases. The high prevalence of overweight and obesity among postmenopausal women, nearing 50% in countries such as Canada,13 increases their risk for the many adverse consequences of increased adiposity.14
Intervening with physical activity may be particularly appropriate in this population. Physical activity seems to be efficacious for weight stability without intervening on diet, but because of the paucity of large trials in postmenopausal women, it is unclear whether it can lead to a significant reduction in adiposity in this population.1, 15, 16, 17, 18, 19 Numerous observational studies suggest that physical activity reduces the risk of postmenopausal breast cancer,20, 21, 22 and that activity carried out after menopause continues to lead to a reduction in risk.1, 23, 24, 25 This effect may operate through a reduction in adiposity (postmenopausal breast cancer risk could be lowered by as much as 20%26, 27or more28 with at least 10 kg weight loss), but there also seem to be effects over and above a reduction in adiposity.1 Other than alcohol intake, few modifiable breast cancer risk factors are known.29
The Alberta Physical Activity and Breast Cancer Prevention (ALPHA) Trial was designed to increase the understanding of the biological mechanisms that mediate the inverse association between physical activity and breast cancer risk. Specifically, it used a randomized, controlled design with a large sample size to examine whether an intensive aerobic exercise intervention influenced the primary end points of adiposity and endogenous sex hormone concentrations, as well as the secondary end points of mammographic density, insulin-like growth factors, insulin resistance and circulating markers of obesity and inflammation. We previously reported the results from this trial on the sex hormones outcomes.30 This paper reports the effects of the exercise intervention on both total and abdominal adiposity, as assessed with dual X-ray absorptiometry and computed tomography. It addresses a gap in the scientific literature for randomized controlled trials carried out in postmenopausal women of exercise interventions that have higher volume and longer duration and that quantify adiposity with direct imaging methods.31 The ALPHA trial also assessed the effect of physical activity independent of a dietary intervention on potential breast cancer risk mediators in order to extend scientific understanding of the mechanisms underlying risk reduction.
Methods
Setting and participants
The design and methods for the ALPHA trial have been previously presented.30 In brief, this study was a two-centre, randomized trial carried out in postmenopausal women living in the cities of Calgary and Edmonton in Alberta, Canada. The study and protocol were approved by the institutional review boards at the Alberta Cancer Board, the Universities of Calgary and Alberta, and all participants provided written informed consent. Women from the general population were recruited through targeted mailings to participants in the Alberta Breast Screening Program, posters and brochures distributed to family physicians and media campaigns. Specific eligibility criteria included age 50–74 years, postmenopausal, no previous cancer diagnosis, no major comorbidities, acceptable baseline fitness test, sedentary (<90 min of weekly exercise or, if between 90 and 120 min, having a VO2max level <34 ml kg−1 min−1), able to do unrestricted physical activity,32 normal blood lipid and hormone levels, BMI between 22 and 40 kg m−2, nonsmoker, <14 drinks per week of alcohol, no medications or exogenous hormones that might influence estrogen metabolism and not currently or planning to undertake a weight loss program. A short telephone screen identified eligible women who then attended an information session in which we explained the study, addressed questions and obtained informed consent. Further screening included three questionnaires, a mammogram, physician approval, blood screening and a submaximal fitness test. Once eligibility was assured, participants were randomized. Recruitment into the study began in May 2003 and was completed in June 2006; follow-up was completed by July 2007.
Randomization and blinding
The randomization sequence was created by the study biostatistician (RB) using a random number program in S-plus (version 6 for UNIX/Linux, Insightful Corp., Seattle, WA, USA). Stratification was carried out by center (Calgary, Edmonton) and BMI (<27.5, ⩾27.5 m kg−2), with blocks randomly sized between four and six within strata to ensure an equal balance of study participants randomized to each intervention condition. Numbered sealed opaque envelopes were created that were opened only by the Study Coordinator in Calgary at the time of randomization. All assessors of the primary outcome measures were blinded to group assignment.
Intervention
The exercise prescription was moderate-to-vigorous intensity aerobic exercise for at least 45 min on 5 days per week for 1 year. At least three sessions per week were facility based with on-site exercise trainers and the remaining sessions were home based. Participants wore heart rate monitors (Polar A3, Polar Electro Oy, Kempele, Finland) to ensure that at least half of their total workout time was at 70–80% of their heart rate reserve. They were instructed to warm up for 5 min, cool down for 5–10 min and stretch. The prescription ramped up over the first 3 months starting with three weekly sessions of 15–20 min at 50–60% of the heart rate reserve. Within these general parameters, the program was individualized to the age and fitness level of each participant. Several methods were used to increase success in meeting the exercise prescription, including scheduling of all facility-based sessions and telephone follow-up of missed sessions, plans for vacations and return after illness or injury, group sessions to permit interaction between participants, a comprehensive educational package highlighting issues of relevance to women starting an exercise program, incentives that were awarded when program milestones were reached, regular newsletters and a study website. Adherence was monitored by weekly exercise logs completed by the participants and the trainers. Women in the control group were asked to maintain their regular lifestyle. Both exercise and control participants were instructed not to change their usual diet.
Measures at baseline and 1 year
Data on demographic characteristics and medical and reproductive history were obtained from a self-administered questionnaire at baseline; all other measures were taken at baseline and at 1 year. Past year dietary intake was assessed using the National Cancer Institute's 124-item Diet History Questionnaire previously adapted for use in Canada,33 and occupational, household and recreational physical activity were assessed using the validated Past Year Total Physical Activity Questionnaire.34 A modified Balke treadmill protocol was used to assess physical fitness by estimating maximum oxygen consumption (VO2max) from submaximal exercise intensities. Oxygen consumption at the age-predicted maximum heart rate (that is, 220-age) was estimated by extrapolating from the stage of the test in which the heart rate reached 85% of the age-predicted maximum heart rate using the American College of Sports Medicine metabolic equations for estimating oxygen consumption at the workload of each stage.35
Anthropometric measurements were made in duplicate; if the two measurements were discrepant (that is, not identical), a third measurement was taken and the average of the two closest was used in the analyses. Weight and height measurements were made using a balance beam scale and a stadiometer. Waist and hip circumferences were measured to the nearest 0.1 cm using a metal tape measure. Body mass index was calculated as weight/height2 (kg m−2).
Total body fat and body fat percentage were assessed using whole body dual X-ray absorptiometry scans. In Calgary at the Foothills Medical Centre, scans were carried out on a Hologic QDR 4500W scanner in whole body mode and analyzed with software version 11.2.1 (Hologic Inc, Bedford, MA, USA). In Edmonton at the University of Alberta, scans were carried out on a Lunar Prodigy scanner in either standard mode or thick mode and analyzed with enCORE Software 6.70.01 before November 2004 and 8.60 after November 2004 (Lunar General Electric Medical Systems, Madison, WI, USA). Percent body fat was calculated as 100% × (fat mass/(fat mass+lean mass)). Fifteen scans that were analyzed with both software versions showed that for percent body fat, the correlation was very high (r=0.9997), with a mean absolute difference of 0.01. A variable composition phantom (Bio-Imaging VCP, Newton, PA, USA) was scanned at both sites at the initiation and completion of the study and determined that no standardization was necessary for comparability; the scans of the phantom differed by less than 0.6%.
Intra-abdominal and subcutaneous fat were measured with a single computed tomography slice at the umbilicus. Scans were carried out at the Alberta Cancer Board facilities; in Calgary using a PQ5000 VisionMaster CT scanner (Marconi, Cleveland, OH, USA) and in Edmonton using a MX8000 multi-slice CT Scanner (Phillips Medical Systems, Cleveland, OH, USA). The study radiologist (TT) used image analysis software from Philips Medical Systems on a workstation (Silicone Graphics Inc., Sunnyvale, CA, USA) to identify and demarcate the thresholds between the subcutaneous and intra-abdominal area. The reliability of the reading of the computed tomography scans was very high (intraclass correlation >0.99).
Sample size
The sample size needed to detect differences in the primary end points of body composition and endogenous estrogen concentrations were calculated based on comparing the means of two independent samples.36 As a smaller effect size was anticipated for estrogen outcomes, the sample size was set at 150 participants per group to detect changes of 10–20% in these outcomes, with 80% power at an α=0.05. This sample size provided a power of >99% to detect anticipated changes of 5–10% in the adiposity outcomes.15, 37 To allow for study dropouts, 160 participants were enrolled per group.
Statistical analyses
The main analyses included participants with complete data at baseline and at 1 year, keeping individuals in the group to which they were randomized regardless of their adherence. The difference in the mean change in the outcomes from baseline to 1 year between the exercise and control groups was tested with two-sample t-tests. For comparison, the analyses were repeated to include the few participants with missing outcome data by assuming that no change occurred in the outcomes. We also examined the statistical significance of the interaction terms between intervention group and the stratification variables for randomization, study center and baseline BMI (⩽27.5, >27.5 kg m−2). In a post hoc analysis, the mean changes from baseline to 1 year was compared between controls and three groups of exercisers on the basis of categories predefined by public health guidelines and the prescription in this study (<150 min per week, 150–225 min per week and >225 min per week).1, 38, 39 All statistical tests were two-sided with a level of significance set at 0.05. Analyses were carried out using SAS (Version 9.1, SAS Institute Inc., Cary, NC, USA).
Results
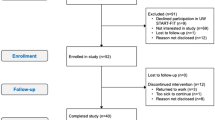
Of 3454 women assessed for eligibility, 1965 did not meet the inclusion criteria, 274 were excluded for other reasons, 895 refused participation and 320 women were randomized, with 165 women from Edmonton and 155 from Calgary (Figure 1). Baseline characteristics of the two groups were similar (Table 1). Nine women were lost to follow-up, one died for reasons unrelated to the trial and eight were no longer interested or too busy. Over the course of the trial no adverse events or side effects were reported in women randomized to the exercise intervention.
Women in the exercise group completed an average of 3.6 sessions per week (s.d.=1.3) for a mean duration of 178.5 min (s.d.=76.1).30 Objectively measured average heart rate was 62.2% (s.d.=9.8) of the estimated heart rate reserve. As assessed using the Past Year Total Physical Activity Questionnaire, 23% (37/160) of controls reported at least 150 min per week of total activity over the intervention year; however, recreational activity increased far less in controls than in exercisers (3.2 vs 20.2 MET-hours per week; P<0.001).40, 41 Furthermore, aerobic fitness increased significantly more in exercisers than in controls (3.9 vs 0.7 ml kg−1 min−1; P<0.001, corresponding to a per cent change of 14.2 vs 2.6%). Mean energy intake decreased among controls relative to exercisers (161 vs 45 kcal per day; P=0.01).
The mean decrease in adiposity between baseline and 1 year was significantly greater in exercisers relative to the controls (P<0.001) (Table 2). Exercisers lost 2.3 kg body weight, corresponding to a 3.0% decrease, whereas controls lost 0.5 kg, equal to a 0.6% decrease. The body weight lost largely constituted of body fat; exercisers lost 2.4 kg body fat, corresponding to a 7.7% decrease, whereas controls lost 0.4 kg, equal to a 1.3% decrease. Abdominal fat area decreased in exercisers, with a mean of 48.5 cm2, of which 16.5 cm2 was intra-abdominal fat area; controls also experienced a small mean decrease in abdominal fat area (9.6 cm2) of which 1.6 cm2 was intra-abdominal fat area. The only nonstatistically significant difference was in lean body mass, which did not decrease among exercisers, but did decrease by 0.1 kg in the control group. If the few participants with missing outcome data were included by assuming no change, or if the baseline value of the outcome measure and covariates were included, the results were similar (data not shown).
The association between the intervention and change in adiposity was similar in each BMI stratum (P-value for interaction terms>0.05; data not shown). The results stratified by study center suggested that greater differences between exercisers and controls were achieved in Edmonton than in Calgary (data not shown). Although women in Edmonton were heavier than women in Calgary, as noted above, baseline adiposity did not modify the effect of the intervention. In addition, the exercise group in Edmonton completed more facility-based sessions per week than exercisers in Calgary (2.7 vs 2.1; P<0.001) and exercised at higher intensity levels (rate of perceived exertion 13.5 vs 11.8; P<0.001).
Greater decreases in adiposity were achieved with a greater mean duration spent at each week exercising (Figure 2). Compared with controls, whose percentage decrease in body fat was 1.3%, the percentage decrease in exercisers with a mean weekly duration <150, 150–225 and ⩾225 min was 2.5%, 8.1 and 11.4%, respectively. An even greater decrease in intra-abdominal fat was seen across these four groups (1.6 vs 10.4 vs 15.0 vs 24.5%).
Using categories suggested for clinical relevance (Table 3),42 in this study, weight loss >5% was achieved in 28% (43/152) of the exercisers compared with 14% (21/155) of the controls. Conversely, weight gain (>3%) was seen in 8% (12/152) of the exercisers compared with 12% (19/155) of the controls. The likelihood of clinically relevant weight loss increased with greater duration of exercise.
Discussion
This yearlong aerobic exercise intervention among postmenopausal women resulted in statistically significant reductions in overall and abdominal adiposity without any intervention to change dietary intake. Furthermore, women in the exercise group, who achieved a higher duration of physical activity, experienced greater average decreases in adiposity. The combination of facility- and home-based program was achievable and resulted in statistically significant increases in physical activity.
Our study differed from previous randomized trials1, 15, 16, 18, 19, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 of exercise interventions among postmenopausal women that were at least 6 months duration and examined adiposity outcomes. It had a large sample size, used quantitative imaging techniques that are preferable for measuring adiposity, had a low drop-out rate, excellent adherence and used a supervised, high volume of exercise. Of these trials, only two studies carried out by Irwin et al. in the United States15, 56 and Velthuis et al. in the Netherlands47 were sufficiently comparable with ours with respect to study design, exercise volume and outcome measures. Irwin et al.15 found statistically significant decreases in adiposity of a smaller magnitude than that in our trial and a dose–response with increasing exercise adherence, whereas the trial by Velthuis and colleagues56 only found statistically significant decreases for total body fat. The Dose–Response to Exercise in postmenopausal Women trial carried out by Church et al.44, 46 was designed to test the effects of three different doses of exercise on cardiorespiratory fitness over a period of 6 months in sedentary postmenopausal, overweight and obese women with elevated blood pressure. These investigators found statistically significant decreases in waist circumference among exercisers, but not in weight or percent body fat, and no dose–response trend was observed.44, 46 A statistically significant effect might have been observed given a longer duration; alternatively, the absence of a dose–response trend may be explained by compensatory increases in energy intake that increase as the weekly volume of exercise increases.46 In our study, energy intake decreased among controls relative to exercisers, suggesting that at least some compensation by energy intake was made.
In this study and most other exercise studies, weight change is largely accounted for by a loss of fat mass with the preservation of lean mass.15, 16, 43, 49 Although the consensus is that overweight or obese subjects will experience greater changes in adiposity than those of normal weight,1, 15, 16, 57, 58 in our study, we did not find that baseline BMI modified the effect of the intervention on changes in adiposity, possibly because most women were overweight or obese.
A recent comprehensive review concluded that without caloric restriction, aerobic exercise in the range of 13–26 MET-hours per week results in decreases in abdominal adiposity.1 This conclusion is supported by our study in which 17 MET-hours per week on average over the intervention year were expended by the exercisers relative to controls. With this volume of exercise, average reductions in intra-abdominal fat and subcutaneous abdominal fat of 17 and 10%, respectively, were achieved. Other studies including postmenopausal women have also observed reductions in abdominal adiposity with aerobic exercise.15, 16, 17, 18, 44, 59 In addition, we found larger reductions with longer weekly duration of exercise; women who exercised >225 min per week had nearly 25% reductions in intra-abdominal fat observed. This dose–response relationship is supported by previous randomized and nonrandomized trials (reviewed in Ohkawara et al.60), but was not observed in the Dose–Response to Exercise in postmenopausal Women trial.44 Our study and others also suggest that intra-abdominal fat may be lost in a higher proportion than overall fat in response to increased energy expenditure, and that intra-abdominal fat may be decreased even in the absence of significant weight loss or after controlling for change in weight.15, 16, 46, 58, 61 Even after controlling for percent change in total fat mass, exercisers in the current study still achieved a statistically significant change in intra-abdominal fat relative to controls (data not shown).
Lower adiposity, intertwined with other related mechanisms such as levels of endogenous sex hormones, metabolic hormones, growth factors and immune factors, likely has a role in the association between physical activity and breast cancer.62, 63, 64 Abdominal adiposity is etiologically relevant to breast cancer risk;65 positive associations with waist–hip ratio, waist circumference or other measures of central adiposity have been found in most studies of postmenopausal breast cancer risk.3, 66, 67, 68, 69 It remains to be seen whether adding a dietary intervention component has additive effects on reductions in adiposity, particularly on intra-abdominal adiposity.70
The limitations of this study include the lack of compliance amongst controls, as 23% reported >150 min per week of physical activity during the intervention, and the limited generalizability of the sample because of the exclusion criteria, including the presence of comorbid conditions such as diabetes and hypercholesterolemia. A per protocol analysis in which participants in either the exercise or control group achieving <150 min per week were compared with participants in either group achieving ⩾150 min per week indicated that the difference in the weight loss between these groups was almost exactly similar to that between the intervention groups (data not shown). Furthermore, despite instructions not to change dietary energy, 20% of exercisers and 31% of controls decreased their caloric intake >300 kcal per day and 14% of exercisers and 8% of controls increased their caloric intake >300 kcal per day. Although food frequency questionnaires are limited as means of measuring dietary intake, because responses are self-reported and subject to measurement error, we repeated the same measurement at the beginning and end of the trial in both exercisers and controls.
It is of importance to note that this study was designed as an efficacy trial, not an effectiveness study, and future research will evaluate how likely uptake of this level of exercise in postmenopausal women would be and how to develop strategies to enhance adoption and maintenance. Furthermore, the level of exercise that was prescribed for our study population is comparable with that found amongst the highest activity groups in observational studies20, 21 and is also within the range of public health recommendations of exercise for chronic disease reduction.1 Although this study has provided some preliminary evidence on how aerobic exercise can be used to decrease adiposity levels that may be associated with cancer risk, it has not addressed the question of the exact dose and type of activity needed for the optimal reduction in adiposity levels. Our preliminary findings from analyses conducted within the exercise arm of the trial need to be substantiated in a future randomized controlled trial that compares different doses of activity in separate arms of the trial and the consequent effect on adiposity and other metabolic hormones. Finally, we recognize that in clinical practice, a multipronged approach should be used to decrease chronic disease risk including intervening on other modifiable lifestyle risk factors. Results from one observational study suggest that postmenopausal breast cancer risk may be reduced by 10% for every 5 kg of weight lost from a woman's highest body weight attained earlier in life,27 which translates into a risk reduction of 4–5% in our exercise group. Higher risk reductions for breast cancer (and other chronic disease associated with obesity) might be attained using a weight loss strategy that incorporates both physical activity and dietary changes.
In conclusion, this trial has addressed a gap identified in the scientific literature for rigorous and well-designed randomized controlled trials of higher volume, longer duration and exercise interventions that quantify using direct imaging methods the impact of exercise on body composition.31 It provides direct empirical evidence that previously sedentary, mostly overweight, postmenopausal women can achieve and sustain high levels of aerobic exercise that result in statistically significant reductions in all measures of adiposity. These levels of change in adiposity through exercise could be beneficial for chronic disease risk reduction.
References
Physical activity guidelines advisory committee. Physical activity guidelines advisory committee report. U.S. Department of Health and Human Services: Washington, D.C., USA, 2008.
Calle EE, Kaaks R . Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004; 4: 579–591.
IARC Working Group. IARC Handbook of Cancer Prevention, Volume 6: Weight Control and Physical Activity. IARC: Lyon, 2002.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M . Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008; 371: 569–578.
Berghofer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN . Obesity prevalence from a European perspective: a systematic review. BMC Public Health 2008; 8: 200.
Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006; 368: 1681–1688.
Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM . Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006; 295: 1549–1555.
Ford ES, Mokdad AH . Epidemiology of obesity in the western hemisphere. J Clin Endocrinol Metab 2008; 93: S1–S8.
Aekplakorn W, Mo-Suwan L . Prevalence of obesity in Thailand. Obes Rev 2009; 10: 589–592.
Lahti-Koski M, Seppanen-Nuijten E, Mannisto S, Harkanen T, Rissanen H, Knekt P et al. Twenty-year changes in the prevalence of obesity among Finnish adults. Obes Rev 2010; 11: 171–176.
Abubakari AR, Lauder W, Agyemang C, Jones M, Kirk A, Bhopal RS . Prevalence and time trends in obesity among adult West African populations: a meta-analysis. Obes Rev 2008; 9: 297–311.
Luo W, Morrison H, de Groh M, Waters C, DesMeules M, Jones-McLean E et al. The burden of adult obesity in Canada. Chronic Dis Can 2007; 27: 135–144.
Slater J, Green C, Sevenhuysen G, O’Neil J, Edginton B . Socio-demographic and geographic analysis of overweight and obesity in Canadian adults using the Canadian Community Health Survey (2005). Chronic Dis Can 2009; 30: 4–15.
Hu FB . Overweight and obesity in women: health risks and consequences. J Womens Health (Larchmt) 2003; 12: 163–172.
Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph RE, Schwartz RS et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA 2003; 289: 323–330.
McTiernan A, Sorensen B, Irwin ML, Morgan A, Yasui Y, Rudolph RE et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring) 2007; 15: 1496–1512.
Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JJ . Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care 2003; 26: 2977–2982.
Slentz CA, Aiken LB, Houmard JA, Bales CW, Johnson JL, Tanner CJ et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol 2005; 99: 1613–1618.
Stefanick ML, Mackey S, Sheehan M, Ellsworth N, Haskell WL, Wood PD . Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N Engl J Med 1998; 339: 12–20.
Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW et al. Physical activity and breast cancer: a systematic review. Epidemiology 2007; 18: 137–157.
Friedenreich CM, Cust AE . Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med 2008; 42: 636–647.
World Cancer Research Fund and the American Institute for Cancer research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. American Institute for Cancer Research: Washington, D.C., USA, 2007.
Cerhan JR, Chiu BC, Wallace RB, Lemke JH, Lynch CF, Torner JC et al. Physical activity, physical function, and the risk of breast cancer in a prospective study among elderly women. J Gerontol A Biol Sci Med Sci 1998; 53A: M251–M256.
Patel AV, Callel EE, Bernstein L, Wu AH, Thun MJ . Recreational physical activity and risk of postmenopausal breast cancer in a large cohort of US women. Cancer Causes Control 2003; 14: 519–529.
McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women's Health Initiative Cohort Study. JAMA 2003; 290: 1331–1336.
Parker ED, Folsom AR . Intentional weight loss and incidence of obesity-related cancers: the Iowa Women's Health Study. Int J Obes Relat Metab Disord 2003; 27: 1447–1452.
Trentham-Dietz A, Newcomb PA, Egan KM, Titus-Ernstoff L, Baron JA, Storer BE et al. Weight change and risk of postmenopausal breast cancer (United States). Cancer Causes Control 2000; 11: 533–542.
Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE . Adult weight change and risk of postmenopausal breast cancer. JAMA 2006; 296: 193–201.
Ballard-Barbash R, Hunsberger S, Alciati MH, Blair SN, Goodwin PJ, McTiernan A et al. Physical activity, weight control, and breast cancer risk and survival: clinical trial rationale and design considerations. J Natl Cancer Inst 2009; 101: 630–643.
Friedenreich CM, Woolcott CG, McTieman A, Ballard-Barbash R, Brant RF, Stanczyk FZ et al. Alberta Physical Activity and Breast Cancer Prevention Trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol 2010; 28: 1458–1466.
Kay SJ, Fiatarone Singh MA . The influence of physical activity on abdominal fat: a systematic review of the literature. Obes Rev 2006; 7: 183–200.
British Columbia Ministry of Health, Canadian Society for Exercise Physiology. Physical Activity Readiness Medical Examination. Canadian Society for Exercise Physiology: Ottawa, Ontario, 2002.
Csizmadi I, Kahle L, Ullman R, Dawe U, Zimmerman T, Friedenreich CM et al. Adaptation and evaluation of the National Cancer Institute's Dietary History Questionnaire and nutrient database for use in Canadian Populations. Public Health Nutr 2007; 10: 88–96.
Friedenreich CM, Courneya KS, Neilson HK, Matthews CE, Willis G, Irwin M et al. Reliability and validity of the Past Year Total Physical Activity Questionnaire. Am J Epidemiol 2006; 163: 959–970.
American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription, 6th edn, Lippincott Williams and Wilkins: Philadelphia, PA, 2000.
Rosner B . Fundamentals of Biostatistics, 2nd edn, Duxbury Press: Boston, 1986.
McTiernan A, Tworoger SS, Ulrich CM, Yasui Y, Irwin ML, Rajan KB et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res 2004; 64: 2923–2928.
Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK . American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009; 41: 459–471.
Health Canada. Canada's Physical Activity Guide to Healthy Active Living. http://www.phac-aspc.gc.ca/pau-uap/paguide/index.html 2007.
Ainsworth BE, Haskell WL, Leon AS, Jacobs Jr DR, Montoye HJ, Sallis JF et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993; 25: 71–80.
Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000; 32: S498–S504.
Stevens J, Truesdale KP, McClain JE, Cai J . The definition of weight maintenance. Int J Obes (Lond) 2006; 30: 391–399.
Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med 2009; 169: 122–131.
Church TS, Earnest CP, Skinner JS, Blair SN . Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA 2007; 297: 2081–2091.
Asikainen TM, Miilunpalo S, Oja P, Rinne M, Pasanen M, Uusi-Rasi K et al. Randomised, controlled walking trials in postmenopausal women: the minimum dose to improve aerobic fitness? Br J Sports Med 2002; 36: 189–194.
Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN . Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One 2009; 4: e4515.
Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 2006; 84: 1033–1042.
Bergström I, Lombardo C, Brinck J . Physical training decreases waist circumference in postmenopausal borderline overweight women. Acta Obstet Gynecol Scand 2009; 88: 308–313.
Santa-Clara H, Szymanski L, Ordille T, Fernhall B . Effects of exercise training on resting metabolic rate in postmenopausal African American and Caucasian women. Metabolism 2006; 55: 1358–1364.
Figueroa A, Going SB, Milliken LA, Blew RM, Sharp S, Teixeira PJ et al. Effects of exercise training and hormone replacement therapy on lean and fat mass in postmenopausal women. J Gerontol A Biol Sci Med Sci 2003; 58: M266–M270.
DiPietro L, Dziura J, Yeckel CW, Neufer PD . Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol 2006; 100: 142–149.
King AC, Tribble DL . The role of exercise in weight regulation in nonathletes. Sports Med 1991; 11: 331–349.
Brooke-Wavell K, Jones PR, Hardman AE . Brisk walking reduces calcaneal bone loss in post-menopausal women. Clin Sci (Lond) 1997; 92: 75–80.
Ready AE, Naimark B, Ducas J, Sawatzky JV, Boreskie SL, Drinkwater DT et al. Influence of walking volume on health benefits in women post-menopause. Med Sci Sports Exerc 1996; 28: 1097–1105.
Hamdorf PA, Withers RT, Penhall RK, Haslam MV . Physical training effects on the fitness and habitual activity patterns of elderly women. Arch Phys Med Rehabil 1992; 73: 603–608.
Velthuis MJ, Schuit AJ, Peeters PH, Monninkhof EM . Exercise program affects body composition but not weight in postmenopausal women. Menopause 2009; 16: 777–784.
Gan SK, Kriketos AD, Ellis BA, Thompson CH, Kraegen EW, Chisholm DJ . Changes in aerobic capacity and visceral fat but not myocyte lipid levels predict increased insulin action after exercise in overweight and obese men. Diabetes Care 2003; 26: 1706–1713.
Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res 2004; 12: 789–798.
Giannopoulou I, Ploutz-Snyder LL, Carhart R, Weinstock RS, Fernhall B, Goulopoulou S et al. Exercise is required for visceral fat loss in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 2005; 90: 1511–1518.
Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I . A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes (Lond) 2007; 31: 1786–1797.
Ross R, Bradshaw AJ . The future of obesity reduction: beyond weight loss. Nat Rev Endocrinol 2009; 5: 319–325.
McTiernan A . Mechanisms linking physical activity with cancer. Nat Rev Cancer 2008; 8: 205–211.
Neilson HK, Friedenreich CM, Brockton NT, Millikan RC . Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev 2009; 18: 11–27.
Campbell KL, McTiernan A . Exercise and biomarkers for cancer prevention studies. J Nutr 2007; 137: 161S–169S.
Lahmann PH, Lissner L, Gullberg B, Olsson H, Berglund G . A prospective study of adiposity and postmenopausal breast cancer risk: the Malmo Diet and Cancer Study. Int J Cancer 2003; 103: 246–252.
Friedenreich CM . Review of anthropometric factors and breast cancer risk. Eur J Cancer Prev 2001; 10: 15–32.
Stephenson GD, Rose DP . Breast cancer and obesity: an update. Nutr Cancer 2003; 45: 1–16.
Ballard-Barbash R, Swanson CA . Body weight: estimation of risk for breast and endometrial cancers. Am J Clin Nutr 1996; 63: 437S–441S.
Ziegler RG . Anthropometry and breast cancer. J Nutr 1997; 127: 924S–928S.
Irwin ML . Randomized controlled trials of physical activity and breast cancer prevention. Exerc Sport Sci Rev 2006; 34: 182–193.
Acknowledgements
This study was funded by a research grant ( no. 017468) from the Canadian Breast Cancer Research Alliance. Dr Friedenreich was funded by career awards from the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research. Dr Courneya is supported by the Canada Research Chairs Program. The study set up was carried out by Kim van der Hoek and Marla Orenstein, and the Study Coordinators were Rosemary Crosby and Ame-Lia Tamburrini. The Fitness Centre Managers were Ben Wilson, Lisa Workman and Diane Cook. The Exercise Trainers were Shannon Hutchins, Kathy Traptow, Shannon Brown, Susan Daniel, Parissa Gillani, Stephanie Sanden, Karen Mackay and Sandra Olsen. Data preparation and analysis was carried out by Sandra Blitz, Sony Brar and Qinggang Wang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Friedenreich, C., Woolcott, C., McTiernan, A. et al. Adiposity changes after a 1-year aerobic exercise intervention among postmenopausal women: a randomized controlled trial. Int J Obes 35, 427–435 (2011). https://doi.org/10.1038/ijo.2010.147
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.147
Keywords
This article is cited by
-
Modifications of 24-h movement behaviors to prevent obesity in retirement: a natural experiment using compositional data analysis
International Journal of Obesity (2023)
-
Dose-response effects of aerobic exercise on adiposity markers in postmenopausal women: pooled analyses from two randomized controlled trials
International Journal of Obesity (2021)
-
Exercise and Cancer Prevention: Current Evidence and Future Directions
Journal of Science in Sport and Exercise (2020)
-
The Effect of Low-Volume High-Intensity Interval Training on Body Composition and Cardiorespiratory Fitness: A Systematic Review and Meta-Analysis
Sports Medicine (2019)
-
Subcutaneous fat loss is greater than visceral fat loss with diet and exercise, weight-loss promoting drugs and bariatric surgery: a critical review and meta-analysis
International Journal of Obesity (2017)