Abstract
The intima-media thickness (IMT) is considered as a surrogate marker for atherosclerotic disease. The aim of this study was to analyze the relationship of carotid IMT with fetuin-A in patients with essential hypertension (EH) and normal renal function. The plasma levels of fetuin-A, interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α) and the biomarker of oxidative stress 8-iso-PGF2alpha were assayed in samples from 105 untreated EH patients. Carotid IMT measurements were also performed. EH was studied overall and after dividing in EH with IMT ⩾ and <0.9 mm. All of the biomarkers were significantly different between the two subgroups, in particular, the fetuin-A level was lower in the patients with an IMT ⩾0.9 mm. In the overall group, the linear analysis of correlation demonstrated that the IMT was significantly inversely correlated with the fetuin-A level (r=−0.40, P<0.0001) and directly with TNF-α (r=0.39, P<0.0001), IL-6 (r=0.38, P<0.0001) and 8-iso-PGF2alpha (r=0.356, P<0.0003). The multiple regression analysis performed that assigned IMT as a dependent variable showed that fetuin-A (β=−0.268, P<0.0001) was independently correlated with the IMT. Receiver-operator curves demonstrated that fetuin-A levels have a predictive power of IMT>0.9 mm (AUC (area under the curve) 0.738, P<0.0001). Our results suggest that in EH, fetuin-A is associated with the IMT independently of oxidative stress and renal function, thus predicting increases in the IMT.
Similar content being viewed by others
Introduction
Subclinical organ damage represents an intermediate stage in the continuum of vascular disease and is a determinant of the overall cardiovascular risk. Arterial stiffness represents an independent predictor for all causes of cardiovascular mortality and morbidity in patients with essential hypertension (EH), diabetes or end-stage renal disease.1, 2 Ultrasound measurements of the intima-media thickness (IMT) are widely used in clinical studies as a surrogate marker for atherosclerotic disease.3, 4 The so-called response-to-injury hypothesis for atherosclerosis states that the initial damage involves the endothelium, with increased oxidative stress leading to endothelial activation and dysfunction. Moreover, atherosclerosis is considered to be an inflammatory disease.5
Fetuin-A is a member of the cystatin superfamily of cysteine protease inhibitors. It is a negative acute-phase glycoprotein produced by the liver and is detectable in the serum. Fetuin-A is an endogenous inhibitor of the insulin receptor tyrosine kinase, having an unclarified role in diabetes mellitus. Recently, a cross-sectional study demonstrated that lower fetuin-A levels were associated with macrovascular complications in high-risk type 2 diabetes patients6 and with the severity of atherosclerosis in patients with peripheral vascular disease and normal renal function.7 Fetuin-A is reported to be a systematically acting inhibitor of extraosseous calcification in fetuin-A knockout mice.8, 9
Several studies reported that lower fetuin-A serum levels were associated with mortality and cardiovascular events in cohorts with end-stage renal disease, whereas a population-based study linked high plasma fetuin-A levels to an increased risk of myocardial infarction and ischemic stroke.10 Diabetic patients having carotid plaque were reported to be characterized by low fetuin-A levels,11 yet only limited data are available in patients with normal kidney functions or moderate renal impairment and advanced atherosclerosis.10, 12
The aim of this cross-sectional, observational study was to analyze the relationship of subclinical atherosclerosis, as evaluated by carotid IMT measurements with fetuin-A, and certain biomarkers of both oxidative stress and inflammation in patients having EH and normal renal function who are free from cardiovascular disease.
Methods
In accordance with the Declaration of Helsinki and institutional guidelines, the protocol for this study was approved by the local Ethics Committee, and the subjects were aware of the investigational nature of the study and agreed to participate after giving informed consent.
Study population
We enrolled 105 consecutive EH patients. These patients visited our Nephrology and Hypertension Unit for the management and/or the differential diagnosis of their hypertensive disease. All of the patients were untreated and exhibited normal renal function according to the NKF guidelines.13
Fifty-five healthy, age-matched individuals among our staff were enrolled as the control group. Both the control individuals and hypertensive patients were Caucasian. The glomerular filtration rate was estimated (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.14 Before the inclusion in the study, all of the subjects underwent a routine chemical analysis, including a hemochrome with a white blood cell count transaminase, gamma glutamyltransferase and alkaline phosphatase evaluations.
Patients were defined as hypertensive when their clinical blood pressure was >140/90 mm Hg, and the severity of hypertension was defined according to the ESH guidelines of 2007.15 The clinical blood pressure was calculated as the average of three consecutive measurements using an automatic sphygmomanometer after the subject had been sitting for 5 min. Secondary or complicated forms of hypertension were excluded by a clinical examination and the determination of the serum creatinine, serum and urinary electrolytes, plasma catecholamine levels and renin activity and renal echography.
The exclusion criteria were as follows: age younger than 18 years or older than 70 years; secondary or accelerated-malignant arterial hypertension; a history of transitory ischemic attack or stroke; a history of angina, coronary heart disease or myocardial infarction, heart failure or abnormalities of cardiac rhythm or conduction under pharmacological treatment. Patients with diabetes or acute infection and subjects having liver disease were also excluded from the study. No patients were on statin treatment.
Study protocol
At 0900 hours on the day of the study, the overnight-fasted patients were placed in a supine position, and blood samples were obtained from an indwelling forearm venous catheter to assay the serum creatinine, lipid profile, fetuin-A, interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), as well as isoprostane 8-iso-PGF2alpha as a biomarker of oxidative stress. The basal blood pressure was also measured. All of the patients underwent an echo-color Doppler of the carotid arteries to measure the IMT.
Laboratory methods
The serum creatinine was measured using the Jaffè method (Instrumentation Laboratory Company, Levington, MA, USA). The plasma total cholesterol (TC), triglycerides (TG) and high-density lipoprotein-cholesterol (HDL-C), after phosphotungstic acid precipitation, were measured using standard enzymatic colorimetric procedures (Roche Diagnostics, Basel, Switzerland) using a COBAS MIRA plus autoanalyzer (Roche Diagnostics). The low-density lipoprotein-cholesterol (LDL-C) was calculated by the Fridewald formula: LDL-C (mg dl−1)=TC (mg dl−1)−TG (mg dl−1)/5-HDL-C (mg dl−1).
The levels of fetuin-A, TNF-α, IL-6 and 8-iso-PGF2alpha were measured using a solid-phase-specific sandwich enzyme-linked immunosorbent assay. Standard curves were constructed using the appropriate concentration for each factor. Fetuin-A was measured using a commercial kit (Epitope Diagnostics, San Diego, CA, USA); the sensitivity was 2.5 ng ml−1.
TNF-α was assayed using an Amersham Biosciences kit (Little Chalfont, England). The sensitivity was <5 pg ml−1, and the reproducibility of the intra- and between-assays had a coefficient of variation <10%. IL-6 was assayed using a commercially available enzyme-linked immunosorbent assay kit (Pierce Biotechnology Rockford, IL, USA). The sensitivity, inter-assay and intra-assay coefficients of variation were <1 ng l−1, <10% and <10%. Isoprostane 8-iso-PGF2alpha was analyzed using a commercial kit (Assay Design, Ann Arbor, MI, USA). The sensitivity was 16.3 pg ml−1, and the inter-assay coefficient of variation was less than 9%.
Assessment of the carotid IMT
Carotid ultrasonography to assess the IMT was performed by two investigators trained for this purpose before starting the study. A Logic P5 PRO (General Electric Company, Milan, Italy) ultrasound device paired with a 5–10 MHz multifrequency high-resolution linear transducer was used to perform the measurements of the IMT. The measurements were performed on the common carotid artery after the examination of a longitudinal section of 10 mm at a distance of 1 cm from the bifurcation. The measurements were performed in the proximal wall and in the distal wall in the lateral, anterior and posterior projections by following an axis that was perpendicular to the artery to discriminate two lines: one for the intima–blood interface and the other for the media–adventitia interface. A total of six measurements were obtained for the right carotid artery and six for the left carotid using the average values (average IMT) calculated automatically by the software. The measurements were obtained with the subject lying down, with the head extended and slightly turned opposite to the carotid being examined, following the recommendations of the Manheim Carotid Intima-Media Thickness Consensus.16 The average IMT was considered abnormal when >0.90 mm or when there were atherosclerotic plaques with a diameter of 1.5 mm or a focal increase of 0.5 mm or 50% of the adjacent IMT.16
Statistical analyses
The results are provided as the mean±s.d. The differences between the groups were evaluated using an ANOVA and the Student’s t-test. In the overall group, the univariate associations between the variables were assessed using the Pearson correlation coefficients. Stepwise multiple linear regression analyses were used to test the independent correlations of the average IMT. Initially, the age, sex (M=1; F=0), clinical (or 24 h) systolic and diastolic blood pressures, serum glucose, smoking habits, uric acid, body mass index, eGFR and CKD-EPI, LDL and HDL cholesterol levels were included in the models. Subsequently, we ran the models again, adding the fetuin-A, TNF-α, IL-6 and 8-iso-PGF2alpha levels after the exclusion of the parameters not associated with the IMT in the preliminary analyses.
Receiver-operator curves (ROCs) were built to assess the power of fetuin-A to predict an IMT >0.9 mm in the overall group. The curves were built using the normal transformed variables of biomarkers with a normal signal-to-noise model. The biomarker values were ranked in deciles, to obtain 10 points to build the ROCs. The ROC areas and D-prime values were considered to be estimators of the predictive power. The statistical analyses were performed using the SYSTAT DATA software package, version 5.2 (Systat, Evanston, IL, USA) and MedCalc software (Mariakerke, Belgium).
Results
Table 1 gives the demographic and clinical data for the 105 hypertensive patients and 55 control individuals. None of the patients exhibited significant hemodynamic carotid stenosis (>50%). The mean plasma levels of fetuin-A, 8-iso-PGF2alpha and of the other biomarkers of inflammation observed in the hypertensive subgroups with IMTs ⩾ and <0.9 mm are shown in Table 2. All of the biomarkers were significantly different between the two subgroups, in particular, the fetuin-A level was lower in patients with an IMT ⩾0.9 mm.
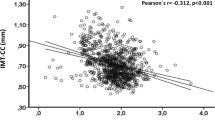
In the overall group, the linear analysis of correlation demonstrated that the IMT was significantly inversely correlated with fetuin-A (r=−0.40, P<0.0001; Figure 1) and directly correlated with TNF-α (r=0.39, P<0.0001), IL-6 (r=0.38, P<0.0001) and 8-iso-PGF2 alpha (r=0.356, P<0.0003).
Moreover, fetuin-A correlated significantly and inversely with TNF-α (r=−0.75, P<0.0001), IL-6 (r=−0.5959, P<0.0001) and 8-iso-PGF2alpha (r=−0.72, P<0.0001). No significant correlations between fetuin A with glycemia or the eGFR (r=0.04 and 0.06, respectively) were found.
A trend of an association between fetuin-A and age was observed, but it was not statistically significant (r=−0.169; P=0.08). The average IMT was strongly associated with age (r=0.46; P<0.001). Moreover, after an adjustment for age, the average IMT was significantly higher in men than in women (0.937±0.24 vs. 0.799±0.22 mm; P=0.002).
In the multiple regression analysis considering IMT as a dependent variable in a model comprising age, sex (M=1, F=0), clinical systolic and diastolic blood pressures and serum glucose, uric acid, body mass index, eGFR and CKD-EPI, LDL and HDL cholesterol levels, only the age (β=0.482; P<0.001) and gender (β=0.257; P=0.004) of the patients were associated with the IMT. Similar results were obtained when the clinical BP values were replaced by the corresponding 24-h readings in the multiple regression model. When the multivariate regression analysis was reperformed, including fetuin-A, TNF-α, IL-6 and 8-iso-PGF2alpha, along with age and sex, in the new model, fetuin-A remained significantly associated with the IMT (Table 3). The ROCs demonstrated that the fetuin-A levels have with a predictive power for an IMT >0.9 mm (AUC (area under the curve) 0.738, P<0.0001): fetuin-A values of 0.77 g l−1 predict an IMT >0.9 mm with a 70% sensitivity and a 68% specificity (Figure 2).
Discussion
This study demonstrates that in essential hypertensive patients having a normal eGFR, lower fetuin-A levels appear to be associated with IMT, a surrogate marker of an atherosclerotic burden. Indeed, fetuin-A correlates inversely with the IMT and has a predictive power for the IMT.
Fetuin-A complexes with calcium and phosphorus in the circulation and prevents the precipitation of these minerals in the serum.17 Fetuin-A is regarded as a marker for vascular inflammation and is one of the most potent negative regulators of vascular ossification-calcification.18, 19 In fetuin-A-deficient mice, the aorta was found to be devoid of calcification and fibrosis, whereas the peripheral vessels in the skin and kidney showed evidence of extensive calcification; furthermore, the small artery involvement preceded the impairment of renal function.17, 18, 20
The data presented herein support the relationship between fetuin-A and IMT, suggesting that decreases in the fetuin-A level could have a role in the development of the vascular changes that are peculiar to hypertensive patients; this role appears to be independent of other confounding factors, such as renal function. Nevertheless the results of this study are not an unambiguous demonstration for the causative role of fetuin-A. The functions and regulatory mechanisms of fetuin-A remain to be fully elucidated and seem to differ according to the pathophysiological characteristics of the population studied.5, 9, 10, 21 Caglar et al.21 showed that the fetuin-A levels significantly increased after renal transplantation, that is, after a nearly complete restoration of the renal function. Recently, the same authors22 hypothesized that fetuin-A may be one of the contributing factors for the development of endothelial dysfunction in CKD patients. It is well known that the latter represents the initial step in the development of atherosclerosis, which is recognized as an inflammatory disease.6
It is noteworthy that in the present study, fetuin-A correlated significantly and inversely with IL-6. We previously demonstrated that in chronic kidney disease, even when not severe, the inflammatory processes assayed by the IL-6 plasma levels are increased and linked to endothelial dysfunction, which progressively worsens with the decline of renal function.23 Furthermore, IL-6 emerged as the strongest independent predictor in a study aimed at evaluating the relationship of some biomarkers of inflammation with the burden of coronary artery disease, as detected by coronary angiography.24
In this study, fetuin-A correlated with the IMT, even independently of the lipid profile and oxidative stress. In fact, considering the well-known relationship between atherosclerosis and oxidative stress, one could expect that its biomarker, 8-iso-PGF2alpha, should correlate with the IMT. However, the significant linear correlation was removed by multiple analyses. These observations corroborate the hypothesis of the role of inflammation biomarkers in vascular changes and suggest that in patients at risk for atherosclerotic disease, the fetuin-A level is decreased even in the early stage of vascular disease, before plaque formation and independently of renal dysfunction.
This hypothesis is in agreement with the data by Emoto11 who demonstrated in 416 patients with type 2 diabetes mellitus and without renal dysfunction that the circulating fetuin-A levels were significantly lower in patients with atherosclerotic calcified plaques than in those without.7 Furthermore, the fetuin-A levels were inversely associated with the presence of calcified plaques, and the authors concluded that fetuin-A may inhibit the calcification of atherosclerotic plaques independently of the dialyzed condition.25, 26 Nevertheless, recent data from the Rancho Bernardo Study27 showed that lower fetuin-A levels were independently associated with coronary artery calcification but not with the IMT in community-living individuals free of cardiovascular disease. These results led to the hypothesis that fetuin-A could be associated with subclinical coronary atherosclerosis but not with atherosclerosis of other vascular beds, thus raising the question of whether it is possible that a circulating protein could target a specific vascular bed. Indeed, experimental studies showed a linkage between fetuin-A and several vascular beds.17
This study has some limitations and strengths. The major limitations consist of the small group of subjects and by the characteristics of a cross-sectional study. The strengths include the well-characterized population, the homogeneous population ethnicity and the absence of both previous cardiovascular events and pharmacological treatment.
In conclusion, our results suggest that in EH, fetuin-A is associated with the IMT independently of oxidative stress and renal function and is a predictor of increases in the IMT. Larger studies are required to confirm these observations.
References
Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S . Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 2002; 39: 10–15.
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H., European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27: 2588–2605.
Salonen JT, Salonen R . Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation 1993; 87 (suppl II): II56–II65.
Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE . Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation 1997; 96: 1432–1437.
Ross R . Atherosclerosis—an inflammatory disease. N Engl J Med 1999; 340: 115–126.
Roos M, Oikonomou D, von Eynatten M, Luppa PB, Heemann U, Lutz J, Baumann M, Nawroth PP, Bierhaus A, Humpert PM . Associations of Fetuin-A levels with vascular disease in type 2 diabetes patients with early diabetic nephropathy. Cardiovasc Diabetol 2010; 9: 48.
Szeberin Z, Fehérvári M, Krepuska M, Apor A, Rimely E, Sarkadi H, Széplaki G, Prohászka Z, Kalabay L, Acsády G . Serum fetuin-A levels inversely correlate with the severity of arterial calcification in patients with chronic lower extremity atherosclerosis without renal disease. Int Angiol 2011; 30: 474–50.
Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W . The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 2003; 112: 357–366.
Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, Häring HU, Boeing H, Fritsche A . Plasma fetuin-A levels and the risk of myocardial infarction and ischemic stroke. Circulation 2008; 118: 2555–2562.
Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley MA . Fetuin-A and kidney function in persons with coronary artery disease – data from the Heart and Soul Study. Nephrol Dial Transplant 2006; 21: 2144–2151.
Emoto M, Mori K, Lee E, Kawano N, Yamazaki Y, Tsuchikura S, Morioka T, Koyama H, Shoji T, Inaba M, Nishizawa Y . Fetuin-A and atherosclerotic calcified plaque in patients with type 2 diabetes mellitus. Metabolism 2010; 59: 873–878.
Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W . The serum protein alpha 2- Heremans-Schmid glyco-protein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 2003; 112: 357–366.
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39 (Suppl 1): S1–S266.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612.
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B,, Management of Arterial Hypertension of the European Society of Hypertension, European Society of Cardiology. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25: 1105–1187.
Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Fatar M, Hernandez Hernandez R, Jaff M, Kownator S, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS, Zannad F, Zureik M . Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 2007; 23: 75–80.
Hayden MR, Tyagi SC, Kolb L, Sowers JR, Khanna R . Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: the emerging role of sodium thiosulfate. Cardiovasc Diabetol 2005; 4: 4.
Merx MW, Schäfer C, Westenfeld R, Brandenburg V, Hidajat S, Weber C, Ketteler M, Jahnen-Dechent W . Myocardial stiffness, cardiac remodeling, and diastolic dysfunction in calcification-prone fetuin-A-deficient mice. J Am Soc Nephrol 2005; 16: 3357–3364.
Fiore CE, Celotta G, Politi GG, Di Pino L, Castelli Z, Mangiafico RA, Signorelli SS, Pennisi P . Association of high alpha2-Heremans-Schmid glycoprotein/fetuin concentration in serum and intima-media thickness in patients with atherosclerotic vascular disease and low bone mass. Atherosclerosis 2007; 195: 110–115.
Cottone S, Nardi E, Mulè G, Vadalà A, Lorito MC, Riccobene R, Palermo A, Arsena R, Guarneri M, Cerasola G . Association between biomarker of inflammation and left ventricular hypertrophy in moderate chronic kidney disease. Clin Nephrol 67: 209–216.
Caglar K, Yilmaz MI, Saglam M, Cakir E, Kilic S, Eyileten T, Sonmez A, Oguz Y, Oner K, Ors F, Vural A, Yenicesu M . Endothelial dysfunction and fetuin A levels before and after kidney transplantation. Transplantation 2007; 83: 392–397.
Caglar K, Yilmaz MI, Saglam M, Cakir E, Kilic S, Sonmez A, Eyileten T, Yenicesu M, Oguz Y, Tasar M, Vural A, Ikizler TA, Stenvinkel P, Lindholm B . Serum fetuin-A concentration and endothelial dysfunction in chronic kidney disease. Nephron Clin Pract 2008; 108: c233–c240.
Cottone S, Palermo A, Arsena R, Riccobene R, Guarneri M, Mulè G, Tornese F, Altieri C, Vaccaro F, Previti A, Cerasola G . Relationship of fetuin-A with glomerular filtration rate and endothelial dysfunction in moderate-severe chronic kidney disease. J Nephrol 2010; 23: 62–69.
Noto D, Cottone S, Baldassare Cefalù A, Vadalà A, Barbagallo CM, Rizzo M, Pernice V, Minà M, Fayer F, Cerasola G, Notarbartolo A, Rocco Averna M . Interleukin 6 plasma levels predict with high sensitivity and specificity coronary stenosis detected by coronary angiography. Thromb Haemost 2007; 98: 1362–1367.
Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Böhm R, Metzger T, Wanner C, Jahnen-Dechent W, Floege J . Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality inpatients on dialysis: a cross-sectional study. Lancet 2003; 361: 827–833.
Ix JH, Barrett-Connor E, Wassel CL, Cummins K, Bergstrom J, Daniels LB, Laughlin GA . The association of fetuin-A with subclinical cardiovascular disease in community-dwelling persons. The Rancho Bernardo Study. J Am Coll Cardiol 2011; 58: 2372–2379.
Hermans MM, Brandenburg V, Ketteler M, Kooman JP, van der Sande FM, Boeschoten EW, Leunissen KM, Krediet RT, Dekker FW,, Netherlands cooperative study on the adequacy of Dialysis (NECOSAD). Association of serum fetuin-A levels with mortality in dialysis patients. Kidney Int 2007; 72: 202–207.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Guarneri, M., Geraci, C., Incalcaterra, F. et al. Subclinical atherosclerosis and fetuin-A plasma levels in essential hypertensive patients. Hypertens Res 36, 129–133 (2013). https://doi.org/10.1038/hr.2012.136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2012.136
Keywords
This article is cited by
-
Circulating calcification inhibitors are associated with arterial damage in pediatric patients with primary hypertension
Pediatric Nephrology (2021)
-
The role of fetuin-A in mineral trafficking and deposition
BoneKEy Reports (2015)
-
Associations of fetuin-A and osteoprotegerin with arterial stiffness and early atherosclerosis in chronic hemodialysis patients
BMC Nephrology (2013)
-
Is fetuin-A a biomarker of preclinical atherosclerosis in essential hypertension?
Hypertension Research (2013)