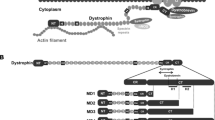
Abstract
The heart is frequently afflicted in muscular dystrophy. In severe cases, cardiac lesion may directly result in death. Over the years, pharmacological and/or surgical interventions have been the mainstay to alleviate cardiac symptoms in muscular dystrophy patients. Although these traditional modalities remain useful, the emerging field of gene therapy has now provided an unprecedented opportunity to transform our thinking/approach in the treatment of dystrophic heart disease. In fact, the premise is already in place for genetic correction. Gene mutations have been identified and animal models are available for several types of muscular dystrophy. Most importantly, innovative strategies have been developed to effectively deliver therapeutic genes to the heart. Dystrophin-deficient Duchenne cardiomyopathy is associated with Duchenne muscular dystrophy (DMD), the most common lethal muscular dystrophy. Considering its high incidence, there has been a considerable interest and significant input in the development of Duchenne cardiomyopathy gene therapy. Using Duchenne cardiomyopathy as an example, here we illustrate the struggles and successes experienced in the burgeoning field of dystrophic heart disease gene therapy. In light of abundant and highly promising data with the adeno-associated virus (AAV) vector, we have specially emphasized on AAV-mediated gene therapy. Besides DMD, we have also discussed gene therapy for treating cardiac diseases in other muscular dystrophies such as limb-girdle muscular dystrophy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ehmsen J, Poon E, Davies K . The dystrophin-associated protein complex. J Cell Sci 2002; 115: 2801–2803.
Ervasti JM . Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. Biochim Biophys Acta 2007; 1772: 108–117.
Dalkilic I, Kunkel LM . Muscular dystrophies: genes to pathogenesis. Curr Opin Genet Dev 2003; 13: 231–238.
Heydemann A, McNally EM . Consequences of disrupting the dystrophin-sarcoglycan complex in cardiac and skeletal myopathy. Trends Cardiovasc Med 2007; 17: 55–59.
Wasala NB, Shin JH, Duan D . The evolution of heart gene delivery vectors. J Gene Med 2011; 13: 557–565.
Carter BJ . Adeno-associated virus and the development of adeno-associated virus vectors: a historical perspective. Mol Ther 2004; 10: 981–989.
Svensson EC, Marshall DJ, Woodard K, Lin H, Jiang F, Chu L et al. Efficient and stable transduction of cardiomyocytes after intramyocardial injection or intracoronary perfusion with recombinant adeno-associated virus vectors. Circulation 1999; 99: 201–205.
Yue Y, Li Z, Harper SQ, Davisson RL, Chamberlain JS, Duan D . Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the Mdx mouse heart. Circulation 2003; 108: 1626–1632.
Hoshijima M, Ikeda Y, Iwanaga Y, Minamisawa S, Date MO, Gu Y et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med 2002; 8: 864–871.
Bostick B, Ghosh A, Yue Y, Long C, Duan D . Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Therapy 2007; 14: 1605–1609.
Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther 2006; 14: 45–53.
Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res 2006; 99: e3–e9.
Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol 2005; 23: 321–328.
Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med 2004; 10: 828–834.
Fine DM, Shin JH, Yue Y, Volkmann D, Leach SB, Smith BF et al. Age-matched comparison reveals early electrocardiography and echocardiography changes in dystrophin-deficient dogs. Neuromuscul Disord 2011; 21: 453–461.
Bostick B, Yue Y, Duan D . Gender influences cardiac function in the mdx model of Duchenne cardiomyopathy. Muscle Nerve 2010; 42: 600–603.
Bostick B, Yue Y, Long C, Duan D . Prevention of dystrophin-deficient cardiomyopathy in twenty-one-month-old carrier mice by mosaic dystrophin expression or complementary dystrophin/utrophin expression. Circ Res 2008; 102: 121–130.
Sakamoto A, Ono K, Abe M, Jasmin G, Eki T, Murakami Y et al. Both hypertrophic and dilated cardiomyopathies are caused by mutation of the same gene, delta-sarcoglycan, in hamster: an animal model of disrupted dystrophin-associated glycoprotein complex. Proc Natl Acad Sci USA 1997; 94: 13873–13878.
Smith BF, Yue Y, Woods PR, Kornegay JN, Shin JH, Williams RR et al. An intronic LINE-1 element insertion in the dystrophin gene aborts dystrophin expression and results in Duchenne-like muscular dystrophy in the corgi breed. Lab Invest 2011; 91: 216–231.
Gregorevic P, Blankinship MJ, Allen JM, Chamberlain JS . Systemic microdystrophin gene delivery improves skeletal muscle structure and function in old dystrophic mdx mice. Mol Ther 2008; 16: 657–664.
Bostick B, Shin J-H, Yue Y, Duan D . AAV-microdystrophin therapy improves cardiac performance in aged female mdx mice. Mol Ther 2011; 19: 1826–1832.
Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS, Metzger JM . Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther 2007; 15: 1086–1092.
Odom GL, Gregorevic P, Allen JM, Chamberlain JS . Gene Therapy of mdx mice with large truncated dystrophins generated by recombination using rAAV6. Mol Ther 2011; 19: 36–45.
Bostick B, Yue Y, Lai Y, Long C, Li D, Duan D . Adeno-associated virus serotype-9 microdystrophin gene therapy ameliorates electrocardiographic abnormalities in mdx mice. Hum Gene Ther 2008; 19: 851–856.
Ghosh A, Yue Y, Shin J-H, Duan D . Systemic trans-splicing AAV delivery efficiently transduces the heart of adult mdx mouse, a model for Duchenne muscular dystrophy. Hum Gene Ther 2009; 20: 1319–1328.
Shin JH, Nitahara-Kasahara Y, Hayashita-Kinoh H, Ohshima-Hosoyama S, Kinoshita K, Chiyo T et al. Improvement of cardiac fibrosis in dystrophic mice by rAAV9-mediated microdystrophin transduction. Gene Therapy 2011; 18: 910–919.
Chamberlain JS . Gene therapy of muscular dystrophy. Hum Mol Genet 2002; 11: 2355–2362.
Duan D . Duchenne muscular dystrophy gene therapy: lost in translation? Res Rep Biol 2011; 2: 31–42.
Duan D . From the smallest virus to the biggest gene: marching towards gene therapy for Duchenne muscular dystrophy. Discov Med 2006; 6: 103–108.
Zhang Y, Duan D . Novel mini-dystrophin gene dual adeno-associated virus vectors restore neuronal nitric oxide synthase expression at the sarcolemma. Hum Gene Ther 2012; 23: 98–103.
Ghosh A, Yue Y, Lai Y, Duan D . A hybrid vector system expands aden-associated viral vector packaging capacity in a transgene independent manner. Mol Ther 2008; 16: 124–130.
Lai Y, Yue Y, Liu M, Ghosh A, Engelhardt JF, Chamberlain JS et al. Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat Biotechnol 2005; 23: 1435–1439.
Duan D, Yan Z, Engelhardt JF . Expanding the capacity of AAV vectors. In: Bloom ME, Cotmore SF, Linden RM, Parrish CR, Kerr JR (eds). Parvoviruses. Hodder Arnold; Distributed in the USA by Oxford University Press: London, New York, 2006, pp 525–532.
Ghosh A, Duan D . Expending adeno-associated viral vector capacity: a tale of two vectors. Biotechnol Genet Eng Rev 2007; 24: 165–177.
Duan D, Yue Y, Engelhardt JF . Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol Ther 2001; 4: 383–391.
Bostick B, Yue Y, Long C, Marschalk N, Fine DM, Chen J et al. Cardiac expression of a mini-dystrophin that normalizes skeletal muscle force only partially restores heart function in aged Mdx mice. Mol Ther 2009; 17: 253–261.
Subramaniam A, Jones WK, Gulick J, Wert S, Neumann J, Robbins J . Tissue-specific regulation of the alpha-myosin heavy chain gene promoter in transgenic mice. J Biol Chem 1991; 266: 24613–24620.
Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med 2006; 12: 787–789.
Aartsma-Rus A, Van Deutekom JC, Fokkema IF, Van Ommen GJ, Den Dunnen JT . Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve 2006; 34: 135–144.
Flanigan KM, Dunn DM, von Niederhausern A, Soltanzadeh P, Gappmaier E, Howard MT et al. Mutational spectrum of DMD mutations in dystrophinopathy patients: application of modern diagnostic techniques to a large cohort. Hum Mutat 2009; 30: 1657–1666.
Koenig M, Beggs AH, Moyer M, Scherpf S, Heindrich K, Bettecken T et al. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet 1989; 45: 498–506.
Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM . An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 1988; 2: 90–95.
Lu QL, Yokota T, Takeda S, Garcia L, Muntoni F, Partridge T . The status of Exon skipping as a therapeutic approach to Duchenne muscular dystrophy. Mol Ther 2011; 19: 9–15.
Goemans NM, Tulinius M, van den Akker JT, Burm BE, Ekhart PF, Heuvelmans N et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med 2011; 364: 1513–1522.
van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med 2007; 357: 2677–2686.
Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L, Torelli S, Anthony K et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet 2011; 378: 595–605.
Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol 2009; 8: 918–928.
Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med 2006; 12: 175–177.
Malerba A, Boldrin L, Dickson G . Long-term systemic administration of unconjugated morpholino oligomers for therapeutic expression of dystrophin by exon skipping in skeletal muscle: implications for cardiac muscle integrity. Nucleic Acid Ther 2011; 21: 293–298.
Wu B, Xiao B, Cloer C, Shaban M, Sali A, Lu P et al. One-year treatment of morpholino antisense oligomer improves skeletal and cardiac muscle functions in dystrophic mdx mice. Mol Ther 2011; 19: 576–583.
Wu B, Lu P, Benrashid E, Malik S, Ashar J, Doran TJ et al. Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino. Gene Therapy 2010; 17: 132–140.
Goyenvalle A, Davies KE . Challenges to oligonucleotides-based therapeutics for Duchenne muscular dystrophy. Skelet Muscle 2011; 1: 8.
Wu B, Moulton HM, Iversen PL, Jiang J, Li J, Spurney CF et al. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc Natl Acad Sci USA 2008; 105: 14814–14819.
Yin H, Lu Q, Wood M . Effective exon skipping and restoration of dystrophin expression by peptide nucleic acid antisense oligonucleotides in mdx mice. Mol Ther 2008; 16: 38–45.
Yin H, Moulton HM, Seow Y, Boyd C, Boutilier J, Iverson P et al. Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum Mol Genet 2008; 17: 3909–3918.
Jearawiriyapaisarn N, Moulton HM, Buckley B, Roberts J, Sazani P, Fucharoen S et al. Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice. Mol Ther 2008; 16: 1624–1629.
Wu B, Li Y, Morcos PA, Doran TJ, Lu P, Lu QL . Octa-guanidine Morpholino Restores Dystrophin Expression in Cardiac and Skeletal Muscles and Ameliorates Pathology in Dystrophic mdx Mice. Mol Ther 2009; 17: 864–871.
Ferlini A, Sabatelli P, Fabris M, Bassi E, Falzarano S, Vattemi G et al. Dystrophin restoration in skeletal, heart and skin arrector pili smooth muscle of mdx mice by ZM2 NP-AON complexes. Gene Therapy 2010; 17: 432–438.
Jearawiriyapaisarn N, Moulton HM, Sazani P, Kole R, Willis MS . Long-term improvement in mdx cardiomyopathy after therapy with peptide-conjugated morpholino oligomers. Cardiovasc Res 2010; 85: 444–453.
Yin H, Moulton HM, Betts C, Seow Y, Boutilier J, Iverson PL et al. A fusion peptide directs enhanced systemic dystrophin exon skipping and functional restoration in dystrophin-deficient mdx mice. Hum Mol Genet 2009; 18: 4405–4414.
Yin H, Moulton HM, Betts C, Merritt T, Seow Y, Ashraf S et al. Functional rescue of dystrophin-deficient mdx mice by a chimeric peptide-PMO. Mol Ther 2010; 18: 1822–1829.
Yin H, Saleh AF, Betts C, Camelliti P, Seow Y, Ashraf S et al. Pip5 transduction peptides direct high efficiency oligonucleotide-mediated dystrophin exon skipping in heart and phenotypic correction in mdx mice. Mol Ther 2011; 19: 1295–1303.
Goyenvalle A, Babbs A, Powell D, Kole R, Fletcher S, Wilton SD et al. Prevention of dystrophic pathology in severely affected dystrophin/utrophin-deficient mice by morpholino-oligomer-mediated exon-skipping. Mol Ther 2010; 18: 198–205.
Amantana A, Moulton HM, Cate ML, Reddy MT, Whitehead T, Hassinger JN et al. Pharmacokinetics, biodistribution, stability and toxicity of a cell-penetrating peptide-morpholino oligomer conjugate. Bioconjug Chem 2007; 18: 1325–1331.
Shin JH, Yue Y, Srivastava A, Smith B, Lai Y, Duan D . A simplified immune suppression scheme leads to persistent micro-dystrophin expression in Duchenne muscular dystrophy dogs. Hum Gene Ther 2012; 23: e-pub ahead of print 14 December 2011; doi:10.1089/hum.2011.147.
Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther 2007; 18: 18–26.
Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther 2007; 15: 1160–1166.
Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N Engl J Med 2010; 363: 1429–1437.
Kaspar RW, Allen HD, Ray WC, Alvarez CE, Kissel JT, Pestronk A et al. Analysis of dystrophin deletion mutations predicts age of cardiomyopathy onset in becker muscular dystrophy. Circ Cardiovasc Genet 2009; 2: 544–551.
Jefferies JL, Eidem BW, Belmont JW, Craigen WJ, Ware SM, Fernbach SD et al. Genetic predictors and remodeling of dilated cardiomyopathy in muscular dystrophy. Circulation 2005; 112: 2799–2804.
Blake DJ, Weir A, Newey SE, Davies KE . Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 2002; 82: 291–329.
Li D, Bareja A, Judge L, Yue Y, Lai Y, Fairclough R et al. Sarcolemmal nNOS anchoring reveals a qualitative difference between dystrophin and utrophin. J Cell Sci 2010; 123: 2008–2013.
Rybakova IN, Humston JL, Sonnemann KJ, Ervasti JM . Dystrophin and utrophin bind actin through distinct modes of contact. J Biol Chem 2006; 281: 9996–10001.
Hirst RC, McCullagh KJ, Davies KE . Utrophin upregulation in Duchenne muscular dystrophy. Acta Myol 2005; 24: 209–216.
Janssen PM, Hiranandani N, Mays TA, Rafael-Fortney JA . Utrophin deficiency worsens cardiac contractile dysfunction present in dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol 2005; 289: H2373–H2378.
Odom GL, Gregorevic P, Allen JM, Finn E, Chamberlain JS . Microutrophin delivery through rAAV6 increases lifespan and improves muscle function in dystrophic dystrophin/utrophin-deficient mice. Mol Ther 2008; 16: 1539–1545.
Tinsley JM, Potter AC, Phelps SR, Fisher R, Trickett JI, Davies KE . Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature 1996; 384: 349–353.
Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail 2008; 14: 355–367.
Shin JH, Bostick B, Yue Y, Hajjar R, Duan D . SERCA2a gene transfer improves electrocardiographic performance in aged mdx mice. J Transl Med 2011; 9: 132.
Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail 2009; 15: 171–181.
Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac Disease (CUPID): a Phase 2 Trial of Intracoronary Gene Therapy of Sarcoplasmic Reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 2011; 124: 304–313.
Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E et al. SUMO1-dependent modulation of SERCA2a in heart failure. Nature 2011; 477: 601–605.
Pepe M, Mamdani M, Zentilin L, Csiszar A, Qanud K, Zacchigna S et al. Intramyocardial VEGF-B167 gene delivery delays the progression towards congestive failure in dogs with pacing-induced dilated cardiomyopathy. Circ Res 2010; 106: 1893–1903.
Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM et al. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation 2011; 124: 1537–1547.
Yue Y, Skimming JW, Liu M, Strawn T, Duan D . Full-length dystrophin expression in half of the heart cells ameliorates beta-isoproterenol-induced cardiomyopathy in mdx mice. Hum Mol Genet 2004; 13: 1669–1675.
Hoogerwaard EM, Bakker E, Ippel PF, Oosterwijk JC, Majoor-Krakauer DF, Leschot NJ et al. Signs and symptoms of Duchenne muscular dystrophy and Becker muscular dystrophy among carriers in The Netherlands: a cohort study. Lancet 1999; 353: 2116–2119.
Grain L, Cortina-Borja M, Forfar C, Hilton-Jones D, Hopkin J, Burch M . Cardiac abnormalities and skeletal muscle weakness in carriers of Duchenne and Becker muscular dystrophies and controls. Neuromuscul Disord 2001; 11: 186–191.
Nolan MA, Jones OD, Pedersen RL, Johnston HM . Cardiac assessment in childhood carriers of Duchenne and Becker muscular dystrophies. Neuromuscul Disord 2003; 13: 129–132.
Holloway SM, Wilcox DE, Wilcox A, Dean JC, Berg JN, Goudie DR et al. Life expectancy and death from cardiomyopathy amongst carriers of Duchenne and Becker muscular dystrophy in Scotland. Heart 2008; 94: 633–636.
Houzelstein D, Lyons GE, Chamberlain J, Buckingham ME . Localization of dystrophin gene transcripts during mouse embryogenesis. J Cell Biol 1992; 119: 811–821.
Hoffman EP, Hudecki MS, Rosenberg PA, Pollina CM, Kunkel LM . Cell and fiber-type distribution of dystrophin. Neuron 1988; 1: 411–420.
Ginjaar IB, Viragh S, Markman MW, van Ommen GJ, Moorman AF . Dystrophin expression in the developing conduction system of the human heart. Microsc Res Tech 1995; 30: 458–468.
Wessels A, Ginjaar IB, Moorman AF, van Ommen GJ . Different localization of dystrophin in developing and adult human skeletal muscle. Muscle Nerve 1991; 14: 1–7.
Merrick D, Stadler LK, Larner D, Smith J . Muscular dystrophy begins early in embryonic development deriving from stem cell loss and disrupted skeletal muscle formation. Dis Model Mech 2009; 2: 374–388.
Gillis JM . Understanding dystrophinopathies: an inventory of the structural and functional consequences of the absence of dystrophin in muscles of the mdx mouse. J Muscle Res Cell Motil 1999; 20: 605–625.
Emery AEH, Muntoni F . Duchenne Muscular Dystrophy, 3rd edn. Oxford University Press: Oxford, New York, 2003, x, 270pp.
Love DR, Byth BC, Tinsley JM, Blake DJ, Davies KE . Dystrophin and dystrophin-related proteins: a review of protein and RNA studies. Neuromuscul Disord 1993; 3: 5–21.
Schofield J, Houzelstein D, Davies K, Buckingham M, Edwards YH . Expression of the dystrophin-related protein (utrophin) gene during mouse embryogenesis. Dev Dyn 1993; 198: 254–264.
Rigoletto C, Prelle A, Ciscato P, Moggio M, Comi G, Fortunato F et al. Utrophin expression during human fetal development. Int J Dev Neurosci 1995; 13: 585–593.
Mora M, Di Blasi C, Barresi R, Morandi L, Brambati B, Jarre L et al. Developmental expression of dystrophin, dystrophin-associated glycoproteins and other membrane cytoskeletal proteins in human skeletal and heart muscle. Brain Res Dev Brain Res 1996; 91: 70–82.
Ng WA, Grupp IL, Subramaniam A, Robbins J . Cardiac myosin heavy chain mRNA expression and myocardial function in the mouse heart. Circ Res 1991; 68: 1742–1750.
Steinhauser ML, Lee RT . Regeneration of the heart. EMBO Mol Med 2011; 3: 701–712.
Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN et al. Transient regenerative potential of the neonatal mouse heart. Science 2011; 331: 1078–1080.
Li F, Wang X, Capasso JM, Gerdes AM . Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol 1996; 28: 1737–1746.
Shin J-H, Bostick B, Yue Y, Duan D . Duchenne cardiomyopathy gene therapy. In: Duan D (ed). Muscle Gene Therapy. Springer Science+Business Media, LLC: New York, 2010, pp 141–162.
Duan D . Challenges and opportunities in dystrophin-deficient cardiomyopathy gene therapy. Hum Mol Genet 2006; 15 (Spec No 2): R253–R261.
Megeney LA, Kablar B, Perry RL, Ying C, May L, Rudnicki MA . Severe cardiomyopathy in mice lacking dystrophin and MyoD. Proc Natl Acad Sci USA 1999; 96: 220–225.
Crisp A, Yin H, Goyenvalle A, Betts C, Moulton HM, Seow Y et al. Diaphragm rescue alone prevents heart dysfunction in dystrophic mice. Hum Mol Genet 2011; 20: 413–421.
Townsend D, Yasuda S, Li S, Chamberlain JS, Metzger JM . Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Mol Ther 2008; 16: 832–835.
Cohen N, Muntoni F . Multiple pathogenetic mechanisms in X linked dilated cardiomyopathy. Heart 2004; 90: 835–841.
Nguyen TM, Ellis JM, Love DR, Davies KE, Gatter KC, Dickson G et al. Localization of the DMDL gene-encoded dystrophin-related protein using a panel of nineteen monoclonal antibodies: presence at neuromuscular junctions, in the sarcolemma of dystrophic skeletal muscle, in vascular and other smooth muscles, and in proliferating brain cell lines. J Cell Biol 1991; 115: 1695–1700.
Rivier F, Robert A, Hugon G, Mornet D . Different utrophin and dystrophin properties related to their vascular smooth muscle distributions. FEBS Lett 1997; 408: 94–98.
Dye WW, Gleason RL, Wilson E, Humphrey JD . Altered biomechanical properties of carotid arteries in two mouse models of muscular dystrophy. J Appl Physiol 2007; 103: 664–672.
Loufrani L, Levy BI, Henrion D . Defect in microvascular adaptation to chronic changes in blood flow in mice lacking the gene encoding for dystrophin. Circ Res 2002; 91: 1183–1189.
Loufrani L, Matrougui K, Gorny D, Duriez M, Blanc I, Levy BI et al. Flow (shear stress)-induced endothelium-dependent dilation is altered in mice lacking the gene encoding for dystrophin. Circulation 2001; 103: 864–870.
Ito K, Kimura S, Ozasa S, Matsukura M, Ikezawa M, Yoshioka K et al. Smooth muscle-specific dystrophin expression improves aberrant vasoregulation in mdx mice. Hum Mol Genet 2006; 15: 2266–2275.
Verma M, Asakura Y, Hirai H, Watanabe S, Tastad C, Fong GH et al. Flt-1 haploinsufficiency ameliorates muscular dystrophy phenotype by developmentally increased vasculature in mdx mice. Hum Mol Genet 2010; 19: 4145–4159.
Blain AM, Straub VW . delta-Sarcoglycan-deficient muscular dystrophy: from discovery to therapeutic approaches. Skelet Muscle 2011; 1: 13.
Gertz EW . Cardiomyopathic Syrian hamster: a possible model of human disease. Prog Exp Tumor Res 1972; 16: 242–260.
Coral-Vazquez R, Cohn RD, Moore SA, Hill JA, Weiss RM, Davisson RL et al. Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell 1999; 98: 465–474.
Hack AA, Lam MY, Cordier L, Shoturma DI, Ly CT, Hadhazy MA et al. Differential requirement for individual sarcoglycans and dystrophin in the assembly and function of the dystrophin-glycoprotein complex. J Cell Sci 2000; 113: 2535–2544.
Holt KH, Lim LE, Straub V, Venzke DP, Duclos F, Anderson RD et al. Functional rescue of the sarcoglycan complex in the BIO 14.6 hamster using delta-sarcoglycan gene transfer. Mol Cell 1998; 1: 841–848.
Ikeda Y, Gu Y, Iwanaga Y, Hoshijima M, Oh SS, Giordano FJ et al. Restoration of deficient membrane proteins in the cardiomyopathic hamster by in vivo cardiac gene transfer. Circulation 2002; 105: 502–508.
Li J, Wang D, Qian S, Chen Z, Zhu T, Xiao X . Efficient and long-term intracardiac gene transfer in delta-sarcoglycan-deficiency hamster by adeno-associated virus-2 vectors. Gene Therapy 2003; 10: 1807–1813.
Toyo-oka T, Kawada T, Xi H, Nakazawa M, Masui F, Hemmi C et al. Gene therapy prevents disruption of dystrophin-related proteins in a model of hereditary dilated cardiomyopathy in hamsters. Heart Lung Circ 2002; 11: 174–181.
Kawada T, Nakazawa M, Nakauchi S, Yamazaki K, Shimamoto R, Urabe M et al. Rescue of hereditary form of dilated cardiomyopathy by rAAV-mediated somatic gene therapy: amelioration of morphological findings, sarcolemmal permeability, cardiac performances, and the prognosis of TO- 2 hamsters. Proc Natl Acad Sci USA 2002; 99: 901–906.
Zhu T, Zhou L, Mori S, Wang Z, McTiernan CF, Qiao C et al. Sustained whole-body functional rescue in congestive heart failure and muscular dystrophy hamsters by systemic gene transfer. Circulation 2005; 112: 2650–2659.
Vitiello C, Faraso S, Sorrentino NC, Di Salvo G, Nusco E, Nigro G et al. Disease rescue and increased lifespan in a model of cardiomyopathy and muscular dystrophy by combined AAV treatments. PLoS One 2009; 4: e5051.
Goehringer C, Rutschow D, Bauer R, Schinkel S, Weichenhan D, Bekeredjian R et al. Prevention of cardiomyopathy in delta-sarcoglycan knockout mice after systemic transfer of targeted adeno-associated viral vectors. Cardiovasc Res 2009; 82: 404–410.
Hoshijima M, Hayashi T, Jeon YE, Fu Z, Gu Y, Dalton ND et al. Delta-sarcoglycan gene therapy halts progression of cardiac dysfunction, improves respiratory failure, and prolongs life in myopathic hamsters. Circ Heart Fail 2011; 4: 89–97.
Chu G, Kranias EG . Phospholamban as a therapeutic modality in heart failure. Novartis Found Symp 2006; 274: 156–171; discussion 172–175, 272–276.
Yang L, Jiang J, Drouin LM, Agbandje-McKenna M, Chen C, Qiao C et al. A myocardium tropic adeno-associated virus (AAV) evolved by DNA shuffling and in vivo selection. Proc Natl Acad Sci USA 2009; 106: 3946–3951.
Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol 2010; 68: 629–638.
Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol 2009; 66: 290–297.
Hermans MC, Pinto YM, Merkies IS, de Die-Smulders CE, Crijns HJ, Faber CG . Hereditary muscular dystrophies and the heart. Neuromuscul Disord 2010; 20: 479–492.
Beynon RP, Ray SG . Cardiac involvement in muscular dystrophies. QJM 2008; 101: 337–344.
Wallace LM, Garwick SE, Harper SQ . RNAi therapy for dominant muscular dystrophies and other myopathies. In: Duan D (ed). Muscle Gene Therapy. Springer Science+Business Media, LLC: New York, 2010, pp 99–115.
Wallace LM, Garwick-Coppens SE, Tupler R, Harper SQ . RNA interference improves myopathic phenotypes in mice over-expressing FSHD Region Gene 1 (FRG1). Mol Ther 2011; 19: 2048–2054.
Acknowledgements
The research in the authors’ laboratory was supported by grants from the National Institutes of Health HL-91883 (DD), Muscular Dystrophy Association (DD), Parent Project Muscular Dystrophy (DD) and Jesse’s Journey: The Foundation for Gene and Cell Therapy (DD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lai, Y., Duan, D. Progress in gene therapy of dystrophic heart disease. Gene Ther 19, 678–685 (2012). https://doi.org/10.1038/gt.2012.10
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2012.10
Keywords
This article is cited by
-
Reducing sarcolipin expression mitigates Duchenne muscular dystrophy and associated cardiomyopathy in mice
Nature Communications (2017)
-
Genome-wide Computational Analysis Reveals Cardiomyocyte-specific Transcriptional Cis-regulatory Motifs That Enable Efficient Cardiac Gene Therapy
Molecular Therapy (2015)
-
Complete restoration of multiple dystrophin isoforms in genetically corrected Duchenne muscular dystrophy patient–derived cardiomyocytes
Molecular Therapy - Methods & Clinical Development (2014)
-
Update on the Treatment of Duchenne Muscular Dystrophy
Current Neurology and Neuroscience Reports (2013)