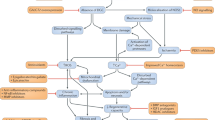
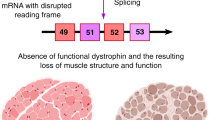
Abstract
Duchenne muscular dystrophy (DMD) is a monogenic muscle-wasting disorder and a priority candidate for molecular and cellular therapeutics. Although rare, it is the most common inherited myopathy affecting children and so has been the focus of intense research activity. It is caused by mutations that disrupt production of the dystrophin protein, and a plethora of drug development approaches are under way that aim to restore dystrophin function, including exon skipping, stop codon readthrough, gene replacement, cell therapy and gene editing. These efforts have led to the clinical approval of four exon skipping antisense oligonucleotides, one stop codon readthrough drug and one gene therapy product, with other approvals likely soon. Here, we discuss the latest therapeutic strategies that are under development and being deployed to treat DMD. Lessons from these drug development programmes are likely to have a major impact on the DMD field, but also on molecular and cellular medicine more generally. Thus, DMD is a pioneer disease at the forefront of future drug discovery efforts, with these experimental treatments paving the way for therapies using similar mechanisms of action being developed for other genetic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hoffman, E. P., Brown, R. H. & Kunkel, L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928 (1987).
Moriuchi, T., Kagawa, N., Mukoyama, M. & Hizawa, K. Autopsy analyses of the muscular dystrophies. Tokushima J. Exp. Med. 40, 83–93 (1993).
Chiang, D. Y. et al. Relation of cardiac dysfunction to rhythm abnormalities in patients with Duchenne or Becker muscular dystrophies. Am. J. Cardiol. 117, 1349–1354 (2016).
Ishikawa, Y. et al. Duchenne muscular dystrophy: survival by cardio-respiratory interventions. Neuromuscul. Disord. 21, 47–51 (2011).
Duan, D., Goemans, N., Takeda, S., Mercuri,E. & Aartsma-Rus, A. Duchenne muscular dystrophy Nat. Rev. Dis. Prim. 7, 14 (2021).
Petrof, B. J., Shrager, J. B., Stedman, H. H., Kelly, A. M. & Sweeney, H. L. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl Acad. Sci. USA 90, 3710–3714 (1993).
Ricotti, V. et al. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 84, 698–705 (2013).
Birnkrant, D. J. et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 17, 347–361 (2018).
Kourakis, S. et al. Standard of care versus new-wave corticosteroids in the treatment of Duchenne muscular dystrophy: can we do better? Orphanet J. Rare Dis. 16, 117 (2021).
Vestergaard, P. et al. Fracture risk in patients with muscular dystrophy and spinal muscular atrophy. J. Rehabil. Med. 33, 150–155 (2001).
Hoffman, E. P. et al. Vamorolone trial in Duchenne muscular dystrophy shows dose-related improvement of muscle function. Neurology 93, e1312–e1323 (2019).
Ervasti, J. M. & Campbell, K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell 66, 1121–1131 (1991).
Rybakova, I. N., Patel, J. R. & Ervasti, J. M. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J. Cell Biol. 150, 1209–1214 (2000).
Spence, H. J., Dhillon, A. S., James, M. & Winder, S. J. Dystroglycan, a scaffold for the ERK-MAP kinase cascade. EMBO Rep. 5, 484–489 (2004).
Dumont, N. A. et al. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med. 21, 1455–1463 (2015).
Roberts, T. C. et al. Multi-level omics analysis in a murine model of dystrophin loss and therapeutic restoration. Hum. Mol. Genet. 24, 6756–6768 (2015).
van Westering, T. L. E. et al. Mutation-independent proteomic signatures of pathological progression in murine models of duchenne muscular dystrophy. Mol. Cell Proteom. 19, 2047–2067 (2020).
Sandonà, D. & Betto, R. Sarcoglycanopathies: molecular pathogenesis and therapeutic prospects. Expert. Rev. Mol. Med. 11, e28 (2009).
Consalvi, S. et al. Histone deacetylase inhibitors in the treatment of muscular dystrophies: epigenetic drugs for genetic diseases. Mol. Med. 17, 457–465 (2011).
Boldrin, L., Zammit, P. S. & Morgan, J. E. Satellite cells from dystrophic muscle retain regenerative capacity. Stem Cell Res. 14, 20–29 (2015).
Meng, J., Bencze, M., Asfahani, R., Muntoni, F. & Morgan, J. E. The effect of the muscle environment on the regenerative capacity of human skeletal muscle stem cells. Skelet. Muscle 5, 11 (2015).
Wang, Y. et al. Dystrophin is a tumor suppressor in human cancers with myogenic programs. Nat. Genet. 46, 601–606 (2014).
Gallia, G. L. et al. Genomic analysis identifies frequent deletions of dystrophin in olfactory neuroblastoma. Nat. Commun. 9, 5410 (2018).
Bladen, C. L. et al. The TREAT-NMD DMD global database: analysis of more than 7,000 duchenne muscular dystrophy mutations. Hum. Mutat. 36, 395–402 (2015).
White, S. J. et al. Duplications in the DMD gene. Hum. Mutat. 27, 938–945 (2006).
Nakamura, A. et al. Deletion of exons 3-9 encompassing a mutational hot spot in the DMD gene presents an asymptomatic phenotype, indicating a target region for multiexon skipping therapy. J. Hum. Genet. 61, 663–667 (2016).
Nakamura, A. et al. Comparison of the phenotypes of patients harboring in-frame deletions starting at exon 45 in the Duchenne muscular dystrophy gene indicates potential for the development of exon skipping therapy. J. Hum. Genet. 62, 459–463 (2017).
Muntoni, F., Torelli, S. & Ferlini, A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2, 731–740 (2003).
England, S. B. et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature 343, 180–182 (1990).
Matsumura, K. et al. Immunohistochemical analysis of dystrophin-associated proteins in Becker/Duchenne muscular dystrophy with huge in-frame deletions in the NH2-terminal and rod domains of dystrophin. J. Clin. Invest. 93, 99–105 (1994).
Monaco, A. P., Bertelson, C. J., Liechti-Gallati, S., Moser, H. & Kunkel, L. M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 2, 90–95 (1988).
Anwar, S., He, M., Lim, K. R. Q., Maruyama, R. & Yokota, T. A genotype-phenotype correlation study of exon skip-equivalent in-frame deletions and exon skip-amenable out-of-frame deletions across the DMD gene to simulate the effects of exon-skipping therapies: a meta-analysis. J. Pers. Med. 11, 46 (2021).
Koenig, M. et al. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am. J. Hum. Genet. 45, 498–506 (1989).
Malhotra, S. et al. Frame-shift deletions in patients with Duchenne and Becker muscular dystrophy. Science 242, 755–759 (1988).
Del Rio-Pertuz, G., Morataya, C., Parmar, K., Dubay, S. & Argueta-Sosa, E. Dilated cardiomyopathy as the initial presentation of Becker muscular dystrophy: a systematic review of published cases. Orphanet J. Rare Dis. 17, 194 (2022).
Aartsma-Rus, A. et al. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum. Mutat. 30, 293–299 (2009).
Syed, Y. Y. Eteplirsen: first global approval. Drugs 76, 1699–1704 (2016).
Heo, Y.-A. Golodirsen: first approval. Drugs 80, 329–333 (2020).
Shirley, M. Casimersen: first approval. Drugs 81, 875–879 (2021).
Komaki, H. et al. Systemic administration of the antisense oligonucleotide NS-065/NCNP-01 for skipping of exon 53 in patients with Duchenne muscular dystrophy. Sci. Transl. Med. 10, eaan0713 (2018).
Clemens, P. R. et al. Safety, tolerability, and efficacy of Viltolarsen in boys with Duchenne muscular dystrophy amenable to exon 53 skipping: a phase 2 randomized clinical trial. JAMA Neurol. 77, 982–991 (2020).
Dhillon, S. Viltolarsen: first approval. Drugs 80, 1027–1031 (2020).
Aartsma-Rus, A. & Goemans, N. A sequel to the eteplirsen saga: eteplirsen is approved in the United States but was not approved in Europe. Nucleic Acid. Ther. 29, 13–15 (2018).
Muntoni, F., Fletcher, S. & Wilton, S. Response to “Railroading at the FDA”. Nat. Biotechnol. 35, 207–209 (2017).
Aartsma-Rus, A. & Krieg, A. M. FDA approves eteplirsen for Duchenne muscular dystrophy: the next chapter in the eteplirsen saga. Nucleic Acid. Ther. 27, 1–3 (2017).
Dowling, J. J. Eteplirsen therapy for Duchenne muscular dystrophy: skipping to the front of the line. Nat. Rev. Neurol. 12, 675–676 (2016).
FDA Briefing Document, Peripheral and Central Nervous System Drugs Advisory Committee Meeting, 22 January 2016, NDA 206488, Eteplirsen (FDA, 2016); https://www.fda.gov/files/advisory%20committees/published/FDA-Briefing-Information-for-the-January-22-2016-Meeting-of-the-Peripheral-and-Central-Nervous-System-Drugs-Advisory-Committee.pdf.
No authors listed. Railroading at the FDA. Nat. Biotechnol. 34, 1078–1078 (2016).
Charleston, J. S. et al. Eteplirsen treatment for Duchenne muscular dystrophy: exon skipping and dystrophin production. Neurology 90, e2146–e2154 (2018).
Servais, L. et al. Long-term safety and efficacy data of golodirsen in ambulatory patients with Duchenne muscular dystrophy amenable to exon 53 skipping: a first-in-human, multicenter, two-part, open-label, phase 1/2 trial. Nucleic Acid. Ther. 32, 29–39 (2022).
Mendell, J. R. et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol. 74, 637–647 (2013).
Mendell, J. R. et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann. Neurol. 79, 257–271 (2016).
Wu, B. et al. Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino. Gene Ther. 17, 132–140 (2010).
Mendell, J. R. et al. Comparison of long-term ambulatory function in patients with Duchenne muscular dystrophy treated with eteplirsen and matched natural history controls. J. Neuromuscul. Dis. 8, 469–479 (2021).
Roberts, T. C., Langer, R. Wood, M. J. A. Advances in oligonucleotide drug delivery Nat. Rev. Drug Discov. 19, 673–694 (2020).
Betts, C. et al. Pip6-PMO, a new generation of peptide-oligonucleotide conjugates with improved cardiac exon skipping activity for DMD treatment. Mol. Ther. Nucleic Acids 1, e38 (2012).
Betts, C. A. et al. Prevention of exercised induced cardiomyopathy following Pip-PMO treatment in dystrophic mdx mice. Sci. Rep. 5, 8986 (2015).
Sarepta therapeutics reports positive clinical results from phase 2 MOMENTUM study of SRP-5051 in patients with duchenne muscular dystrophy amenable to skipping exon 51. Sarepta Therapeutics (5 May 2021); https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-reports-positive-clinical-results-phase-2.
Moulton, H. M. & Moulton, J. D. Morpholinos and their peptide conjugates: therapeutic promise and challenge for Duchenne muscular dystrophy. Biochim. Biophys. Acta 1798, 2296–2303 (2010).
Amantana, A. et al. Pharmacokinetics, biodistribution, stability and toxicity of a cell-penetrating peptide-morpholino oligomer conjugate. Bioconjug. Chem. 18, 1325–1331 (2007).
PepGen reports positive data from phase 1 trial of PGN-EDO51 for the treatment of Duchenne muscular dystrophy. PepGen (28 September 2022); https://investors.pepgen.com/news-releases/news-release-details/pepgen-reports-positive-data-phase-1-trial-pgn-edo51-treatment/.
Kreher, N. et al. P.194 Development of a novel, EEV-conjugated PMO for Duchenne muscular dystrophy. Neuromuscul. Disord. 32, S126 (2022).
Avidity Biosciences Announces Phase 1/2 EXPLORE44TM Trial of AOC 1044 for Duchenne Muscular Dystrophy Mutations Amenable to Exon 44 Skipping (Avidity Biosciences, 2022); https://aviditybiosciences.investorroom.com/2022-10-11-Avidity-Biosciences-Announces-Phase-1-2-EXPLORE44-TM-Trial-of-AOC-1044-for-Duchenne-Muscular-Dystrophy-Mutations-Amenable-to-Exon-44-Skipping.
Desjardins, C. A. et al. Enhanced exon skipping and prolonged dystrophin restoration achieved by TfR1-targeted delivery of antisense oligonucleotide using FORCE conjugation in mdx mice. Nucleic Acids Res. 50, 11401–11414 (2022).
Aoki, Y. et al. Bodywide skipping of exons 45–55 in dystrophic mdx52 mice by systemic antisense delivery. Proc. Natl Acad. Sci. USA 109, 13763–13768 (2012).
Béroud, C. et al. Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum. Mutat. 28, 196–202 (2007).
Wave Life Sciences provides positive update on proof-of-concept study for WVE-N531 in Duchenne muscular dystrophy. GlobeNewswire News Room (WAVE Life Science USA, 2022); https://www.globenewswire.com/news-release/2022/12/19/2576214/0/en/Wave-Life-Sciences-Provides-Positive-Update-on-Proof-of-Concept-Study-for-WVE-N531-in-Duchenne-Muscular-Dystrophy.html.
Kandasamy, P. et al. Control of backbone chemistry and chirality boost oligonucleotide splice switching activity. Nucleic Acids Res. 50, 5443–5466 (2022).
Iwamoto, N. et al. Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat. Biotechnol. 35, 845–851 (2017).
Wan, W. B. et al. Synthesis, biophysical properties and biological activity of second generation antisense oligonucleotides containing chiral phosphorothioate linkages. Nucleic Acids Res. 42, 13456–13468 (2014).
Wave Life Sciences provides update on phase 1b/2a PRECISION-HD trials - Wave Life Sciences. Wave Life Sciences (2021); https://ir.wavelifesciences.com/news-releases/news-release-details/wave-life-sciences-provides-update-phase-1b2a-precision-hd.
Wave Life Sciences announces discontinuation of suvodirsen development for Duchenne muscular dystrophy. Wave Life Sciences (16 December 2019); https://ir.wavelifesciences.com/news-releases/news-release-details/wave-life-sciences-announces-discontinuation-suvodirsen.
Ito, K. et al. Renadirsen, a novel 2’OMeRNA/ENA® chimera antisense oligonucleotide, induces robust exon 45 skipping for dystrophin in vivo. Curr. Issues Mol. Biol. 43, 1267–1281 (2021).
Goyenvalle, A. et al. Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers. Nat. Med. 21, 270–275 (2015).
Zarrouki, F. et al. Partial restoration of brain dystrophin and behavioral deficits by exon skipping in the muscular dystrophy X-linked (mdx) mouse. Ann. Neurol. 92, 213–229 (2022).
De Angelis, F. G. et al. Chimeric snRNA molecules carrying antisense sequences against the splice junctions of exon 51 of the dystrophin pre-mRNA induce exon skipping and restoration of a dystrophin synthesis in Delta 48-50 DMD cells. Proc. Natl Acad. Sci. USA 99, 9456–9461 (2002).
Goyenvalle, A. et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science 306, 1796–1799 (2004).
Simmons, T. R. et al. Pre-clinical dose-escalation studies establish a therapeutic range for U7snRNA-mediated DMD exon 2 skipping. Mol. Ther. Methods Clin. Dev. 21, 325–340 (2021).
Wein, N. et al. Translation from a DMD exon 5 IRES results in a functional dystrophin isoform that attenuates dystrophinopathy in humans and mice. Nat. Med. 20, 992–1000 (2014).
Nationwide Children’s Hospital Announces Restoration of Full-Length Dystrophin Using dup 2 Gene Therapy Approach (Parent Project Muscular Dystrophy, 2022); https://www.parentprojectmd.org/nationwide-childrens-hospital-announces-restoration-of-full-length-dystrophin-using-duplication-2-gene-therapy-approach/.
Barton-Davis, E. R., Cordier, L., Shoturma, D. I., Leland, S. E. & Sweeney, H. L. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J. Clin. Invest. 104, 375–381 (1999).
Wagner, K. R. et al. Gentamicin treatment of Duchenne and Becker muscular dystrophy due to nonsense mutations. Ann. Neurol. 49, 706–711 (2001).
Politano, L. et al. Gentamicin administration in Duchenne patients with premature stop codon. Preliminary results. Acta Myol. 22, 15–21 (2003).
Malik, V. et al. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann. Neurol. 67, 771–780 (2010).
Hayward, R. S. et al. Adverse effects of a single dose of gentamicin in adults: a systematic review. Br. J. Clin. Pharmacol. 84, 223–238 (2018).
Welch, E. M. et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature 447, 87–91 (2007).
Ryan, N. J. Ataluren: first global approval. Drugs 74, 1709–1714 (2014).
Bushby, K. et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve 50, 477–487 (2014).
McDonald, C. M. et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390, 1489–1498 (2017).
No authors listed.Duchenne drug clings on for FDA nodNat. Biotechnol. 35, 999 (2017).
Campbell, C. et al. Meta-analyses of ataluren randomized controlled trials in nonsense mutation Duchenne muscular dystrophy. J. Comp. Eff. Res. 9, 973–984 (2020).
Auld, D. S. et al. Molecular basis for the high-affinity binding and stabilization of firefly luciferase by PTC124. Proc. Natl Acad. Sci. USA 107, 4878–4883 (2010).
McElroy, S. P. et al. A lack of premature termination codon read-through efficacy of PTC124 (Ataluren) in a diverse array of reporter assays. PLoS Biol. 11, e1001593 (2013).
Halbert, C. L., Rutledge, E. A., Allen, J. M., Russell, D. W. & Miller, A. D. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 74, 1524–1532 (2000).
Gruntman, A. M. et al. Gene transfer in skeletal and cardiac muscle using recombinant adeno-associated virus. Curr. Protoc. Microbiol. https://doi.org/10.1002/9780471729259.mc14d03s28 (2013).
Qiao, C., Koo, T., Li, J., Xiao, X. & Dickson, J. G. Gene therapy in skeletal muscle mediated by adeno-associated virus vectors. Methods Mol. Biol. 807, 119–140 (2011).
Gregorevic, P. et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med. 10, 828–834 (2004).
Duan, D. Systemic AAV micro-dystrophin gene therapy for duchenne muscular dystrophy. Mol. Ther. 26, 2337–2356 (2018).
Bourdon, A. et al. Evaluation of the dystrophin carboxy-terminal domain for micro-dystrophin gene therapy in cardiac and skeletal muscles in the DMDmdx rat model. Gene Ther. 29, 520–535 (2022).
Cox, G. A. et al. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature 364, 725–729 (1993).
Potter, R. A. et al. Dose-escalation study of systemically delivered rAAVrh74.MHCK7.micro-dystrophin in the mdx mouse model of duchenne muscular dystrophy. Hum. Gene Ther. 32, 375–389 (2021).
Yue, Y. et al. Safe and bodywide muscle transduction in young adult Duchenne muscular dystrophy dogs with adeno-associated virus. Hum. Mol. Genet. 24, 5880–5890 (2015).
Le Guiner, C. et al. Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat. Commun. 8, 16105 (2017).
Salva, M. Z. et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol. Ther. 15, 320–329 (2007).
Chicoine, L. G. et al. Vascular delivery of rAAVrh74.MCK.GALGT2 to the gastrocnemius muscle of the rhesus macaque stimulates the expression of dystrophin and laminin α2 surrogates. Mol. Ther. 22, 713–724 (2014).
Zygmunt, D. A., Crowe, K. E., Flanigan, K. M. & Martin, P. T. Comparison of serum rAAV serotype-specific antibodies in patients with duchenne muscular dystrophy, becker muscular dystrophy, inclusion body myositis, or GNE myopathy. Hum. Gene Ther. 28, 737–746 (2017).
Mendell, J. R. et al. Assessment of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin in children with duchenne muscular dystrophy: a nonrandomized controlled trial. JAMA Neurol. 77, 1122–1131 (2020).
Willcocks, R. J. et al. Assessment of rAAVrh.74.MHCK7.micro-dystrophin gene therapy using magnetic resonance imaging in children with duchenne muscular dystrophy. JAMA Netw. Open 4, e2031851 (2021).
Mendell, J. et al. A multicenter randomized, double-blind, placebo-controlled, gene-delivery clinical trial of rAAVrh74.MHCK7.micro-dystrophin for Duchenne muscular dystrophy [Abstr.]. Neurology 96 (Suppl. 15), 4478 (2021).
Sarepta Therapeutics announces top-line results for part 1 of study 102 evaluating SRP-9001, its investigational gene therapy for the treatment of Duchenne muscular dystrophy. Sarepta Therapeutics (7 Junuary 2021); https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-top-line-results-part-1-study-102.
Sarepta Therapeutics’ investigational gene therapy SRP-9001 for Duchenne muscular dystrophy demonstrates significant functional improvements across multiple studies. Sarepta Therapeutics (6 July 2022); https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-investigational-gene-therapy-srp-9001.
Sarepta Therapeutics announces that U.S. FDA has accepted for filing and granted priority review for the Biologics License Application for SRP-9001, Sarepta’s gene therapy for the treatment of ambulant individuals with Duchenne muscular dystrophy. Sarepta Therapeutics (28 November 2022); https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-us-fda-has-accepted-filing-and.
Sarepta Therapeutics announces FDA approval of ELEVIDYS, the first gene therapy to treat Duchenne muscular dystrophy. Sarepta Therapeutics (22 June 2023); https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-fda-approval-elevidys-first-gene.
Philippidis, A. After patient death, FDA places hold on Pfizer Duchenne muscular dystrophy gene therapy trial. Hum. Gene Ther. 33, 111–115 (2022).
Philippidis, A. Pfizer eyes resuming phase III enrollment, investigates phase Ib death tied to Duchenne muscular dystrophy candidate. Hum. Gene Ther. 33, 215–217 (2022).
Pfizer’s new phase 1b results of gene therapy in ambulatory boys with Duchenne muscular dystrophy (DMD) support advancement into pivotal phase 3 study. Pfizer (15 May 2020); https://www.pfizer.com/news/press-release/press-release-detail/pfizers-new-phase-1b-results-gene-therapy-ambulatory-boys.
Philippidis, A. FDA lifts clinical hold on Pfizer DMD gene therapy linked to patient death. GEN - Genetic Engineering and Biotechnology News (28 April 2022); https://www.genengnews.com/topics/genome-editing/gene-therapy/fda-lifts-clinical-hold-on-pfizer-dmd-gene-therapy-linked-to-patient-death/.
Pfizer announces amendment to ongoing gene therapy phase III trial. Parent Project Muscular Dystrophy (28 September 2021); https://www.parentprojectmd.org/pfizer-announces-amendment-to-ongoing-gene-therapy-phase-iii-trial/.
Collaborative analysis reveals ‘class effect’ in DMD safety issues. BioSpace (19 May 2022); https://www.biospace.com/article/pfizer-sarepta-genethon-solid-bio-team-up-to-fight-dmd/.
Solid Biosciences provides SGT-001 program update. Solid Biosciences (19 November 2019); https://www.solidbio.com/about/media/press-releases/solid-biosciences-provides-sgt-001-program-update.
Solid Biosciences announces FDA lifts clinical hold on IGNITE DMD clinical trial. Solid Biosciences (1 October 2020); https://www.solidbio.com/about/media/press-releases/solid-biosciences-announces-fda-lifts-clinical-hold-on-ignite-dmd-clinical-trial.
Solid Biosciences reports fourth quarter and full-year 2021 financial results and 2-year efficacy and safety data from the ongoing phase I/II IGNITE DMD clinical trial of SGT-001. Solid Biosciences (14 March 2022); https://www.solidbio.com/about/media/press-releases/solid-biosciences-reports-fourth-quarter-and-full-year-2021-financial-results-and-2-year-efficacy-and-safety-data-from-the-ongoing-phase-i-ii-ignite-dmd-clinical-trial-of-sgt-001.
High-dose AAV gene therapy deaths. Nat. Biotechnol. 38, 910 (2020).
Lysogene confirms child’s death in phase II/III gene therapy trial. GEN (26 October 2020); https://www.genengnews.com/news/lysogene-confirms-childs-death-in-phase-ii-iii-gene-therapy-trial/.
Reuters. Novartis reports Zolgensma caused two deaths from liver failure. Reuters (11 August 2022); https://www.reuters.com/business/healthcare-pharmaceuticals/novartis-reports-zolgensma-caused-two-deaths-liver-failure-2022-08-11/.
Hinderer, C. et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum. Gene Ther. 29, 285–298 (2018).
Hale, C. Solid Bio Sees Yet Another Clinical Hold for its DMD Gene Therapy (Fierce Biotech, 2019); https://www.fiercebiotech.com/biotech/solid-bio-sees-yet-another-clinical-hold-for-its-dmd-gene-therapy.
Jeune, V. L., Joergensen, J. A., Hajjar, R. J. & Weber, T. Pre-existing anti–adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods 24, 59–67 (2013).
Mendell, J. R. et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N. Engl. J. Med. 363, 1429–1437 (2010).
Li, N. et al. The effect of immunomodulatory treatments on anti-Dystrophin immune response after AAV gene therapy in dystrophin deficient mdx mice. J. Neuromuscul. Dis. 8, S325–S340 (2021).
Rivera, V. M. et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood 105, 1424–1430 (2005).
Penaud-Budloo, M. et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J. Virol. 82, 7875–7885 (2008).
Le Hir, M. et al. AAV genome loss from dystrophic mouse muscles during AAV-U7 snRNA-mediated exon-skipping therapy. Mol. Ther. 21, 1551–1558 (2013).
Das, A. et al. Epigenetic silencing of recombinant adeno-associated virus genomes by NP220 and the HUSH complex. J. Virol. 96, e0203921 (2022).
Mollard, A. et al. Muscle regeneration affects adeno associated virus 1 mediated transgene transcription. Sci. Rep. 12, 9674 (2022).
Morgan, J. E., Hoffman, E. P. & Partridge, T. A. Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse. J. Cell Biol. 111, 2437–2449 (1990).
Partridge, T. A., Morgan, J. E., Coulton, G. R., Hoffman, E. P. & Kunkel, L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature 337, 176–179 (1989).
Garcia, S. M. et al. High-yield purification, preservation, and serial transplantation of human satellite cells. Stem Cell Rep. 10, 1160–1174 (2018).
Ferrari, G. et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279, 1528–1530 (1998).
Gussoni, E. et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401, 390–394 (1999).
Cossu, G. et al. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol. Med. 7, 1513–1528 (2015).
Dellavalle, A. et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 9, 255–267 (2007).
Torrente, Y. et al. Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transpl. 16, 563–577 (2007).
Young, C. S. et al. A single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell 18, 533–540 (2016).
Skuk, D. et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J. Neuropathol. Exp. Neurol. 65, 371–386 (2006).
Motohashi, N., Shimizu-Motohashi, Y., Roberts, T. C. & Aoki, Y. Potential therapies using myogenic stem cells combined with bio-engineering approaches for treatment of muscular dystrophies. Cells 8, 1066 (2019).
Skuk, D. & Tremblay, J. P. Myoblast transplantation: the current status of a potential therapeutic tool for myopathies. J. Muscle Res. Cell Motil. 24, 285–300 (2003).
Taylor, M. et al. Cardiac and skeletal muscle effects in the randomized HOPE-Duchenne trial. Neurology 92, e866–e878 (2019).
McDonald, C. M. et al. Repeated intravenous cardiosphere-derived cell therapy in late-stage Duchenne muscular dystrophy (HOPE-2): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 399, 1049–1058 (2022).
Hanson, B., Wood, M. J. A. & Roberts, T. C. Molecular correction of Duchenne muscular dystrophy by splice modulation and gene editing. RNA Biol. 18, 1048–1062 (2021).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Tabebordbar, M. et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351, 407–411 (2016).
Nelson, C. E. et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407 (2016).
Long, C. et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351, 400–403 (2016).
Amoasii, L. et al. Single-cut genome editing restores dystrophin expression in a new mouse model of muscular dystrophy. Sci. Transl. Med. 9, eaan8081 (2017).
Arnett, A. L. et al. Adeno-associated viral (AAV) vectors do not efficiently target muscle satellite cells. Mol. Ther. Methods Clin. Dev. 1, 14038 (2014).
Nance, M. E. et al. AAV9 edits muscle stem cells in normal and dystrophic adult mice. Mol. Ther. 27, 1568–1585 (2019).
Kwon, J. B. et al. In vivo gene editing of muscle stem cells with adeno-associated viral vectors in a mouse model of Duchenne muscular dystrophy. Mol. Ther. Methods Clin. Dev. 19, 320–329 (2020).
Ousterout, D. G. et al. Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat. Commun. 6, 6244 (2015).
Liao, H.-K. et al. In vivo target gene activation via CRISPR/Cas9-mediated trans-epigenetic modulation. Cell 171, 1495–1507.e15 (2017).
Gilbert, L. A. et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451 (2013).
To Our Community: An Update On Our CRD-TMH-001 Clinical Trial (Cure Rare Disease, 2022); https://www.cureraredisease.org/blog-posts/to-our-community-an-update-on-our-crd-tmh-001-clinical-trial.
Lek, A. et al. Unexpected death of a Duchenne muscular dystrophy patient in an N-of-1 Trial of rAAV9-delivered CRISPR-transactivator. Preprint at https://doi.org/10.1101/2023.05.16.23289881 (2023).
Pipeline and Progress (Cure Rare Disease, accessed 2023); https://www.cureraredisease.org/our-approach/pipeline-and-progress#section_1.
Hanson, B. et al. Non-uniform dystrophin re-expression after CRISPR-mediated exon excision in the dystrophin/utrophin double-knockout mouse model of DMD. Mol. Ther. - Nucleic Acids 30, 379–397 (2022).
Simhadri, V. L. et al. Prevalence of pre-existing antibodies to CRISPR-associated nuclease Cas9 in the USA population. Mol. Ther. Methods Clin. Dev. 10, 105–112 (2018).
Charlesworth, C. T. et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med. 25, 249–254 (2019).
Wagner, D. L. et al. High prevalence of Streptococcus pyogenes Cas9-reactive T cells within the adult human population. Nat. Med. 25, 242–248 (2019).
Bengtsson, N. E. et al. Muscle-specific CRISPR/Cas9 dystrophin gene editing ameliorates pathophysiology in a mouse model for Duchenne muscular dystrophy. Nat. Commun. 8, 14454 (2017).
Zhang, Y. et al. CRISPR-Cpf1 correction of muscular dystrophy mutations in human cardiomyocytes and mice. Sci. Adv. 3, e1602814 (2017).
Porto, E. M., Komor, A. C., Slaymaker, I. M. & Yeo, G. W. Base editing: advances and therapeutic opportunities. Nat. Rev. Drug. Discov. 19, 839–859 (2020).
Xu, L. et al. Efficient precise in vivo base editing in adult dystrophic mice. Nat. Commun. 12, 3719 (2021).
Dianov, G. L. & Hübscher, U. Mammalian base excision repair: the forgotten archangel. Nucleic Acids Res. 41, 3483–3490 (2013).
Ryu, S.-M. et al. Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat. Biotechnol. 36, 536–539 (2018).
Chemello, F. et al. Precise correction of Duchenne muscular dystrophy exon deletion mutations by base and prime editing. Sci. Adv. 7, eabg4910 (2021).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).
Blake, D. J., Tinsley, J. M. & Davies, K. E. Utrophin: a structural and functional comparison to dystrophin. Brain Pathol. 6, 37–47 (1996).
Tinsley, J. M. et al. Primary structure of dystrophin-related protein. Nature 360, 591–593 (1992).
Love, D. R. et al. An autosomal transcript in skeletal muscle with homology to dystrophin. Nature 339, 55–58 (1989).
Anthony, K. et al. Biochemical characterization of patients with in-frame or out-of-frame DMD deletions pertinent to exon 44 or 45 skipping. JAMA Neurol. 71, 32–40 (2014).
Matsumura, K., Ervasti, J. M., Ohlendieck, K., Kahl, S. D. & Campbell, K. P. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature 360, 588–591 (1992).
Deconinck, A. E. et al. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell 90, 717–727 (1997).
Grady, R. M. et al. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 90, 729–738 (1997).
Tinsley, J. et al. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat. Med. 4, 1441–1444 (1998).
Squire, S. et al. Prevention of pathology in mdx mice by expression of utrophin: analysis using an inducible transgenic expression system. Hum. Mol. Genet. 11, 3333–3344 (2002).
Fisher, R. et al. Non-toxic ubiquitous over-expression of utrophin in the mdx mouse. Neuromuscul. Disord. 11, 713–721 (2001).
Song, Y. et al. Non-immunogenic utrophin gene therapy for the treatment of muscular dystrophy animal models. Nat. Med. 25, 1505–1511 (2019).
Chancellor, D. R. et al. Discovery of 2-arylbenzoxazoles as upregulators of utrophin production for the treatment of Duchenne muscular dystrophy. J. Med. Chem. 54, 3241–3250 (2011).
Tinsley, J. M. et al. Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PLoS ONE 6, e19189 (2011).
Muntoni, F. et al. A phase 1b trial to assess the pharmacokinetics of ezutromid in pediatric Duchenne muscular dystrophy patients on a balanced diet. Clin. Pharmacol. Drug Dev. 8, 922–933 (2019).
Wilkinson, I. V. L. et al. Chemical proteomics and phenotypic profiling identifies the aryl hydrocarbon receptor as a molecular target of the utrophin modulator ezutromid. Angew. Chem. Int. Ed. 59, 2420–2428 (2020).
Guiraud, S. et al. Second-generation compound for the modulation of utrophin in the therapy of DMD. Hum. Mol. Genet. 24, 4212–4224 (2015).
Tinsley, J. M. et al. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature 384, 349–353 (1996).
Deconinck, N. et al. Expression of truncated utrophin leads to major functional improvements in dystrophin-deficient muscles of mice. Nat. Med. 3, 1216–1221 (1997).
Odom, G. L., Gregorevic, P., Allen, J. M., Finn, E. & Chamberlain, J. S. Microutrophin delivery through rAAV6 increases lifespan and improves muscle function in dystrophic dystrophin/utrophin-deficient mice. Mol. Ther. 16, 1539–1545 (2008).
Sengupta, K. et al. Genome editing-mediated utrophin upregulation in Duchenne muscular dystrophy stem cells. Mol. Ther. Nucleic Acids 22, 500–509 (2020).
Pisani, C. et al. Utrophin up-regulation by artificial transcription factors induces muscle rescue and impacts the neuromuscular junction in mdx mice. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 1172–1182 (2018).
Li, D. et al. Sarcolemmal nNOS anchoring reveals a qualitative difference between dystrophin and utrophin. J. Cell Sci. 123, 2008–2013 (2010).
Belanto, J. J. et al. Microtubule binding distinguishes dystrophin from utrophin. Proc. Natl Acad. Sci. USA 111, 5723–5728 (2014).
Markati, T., De Waele, L., Schara-Schmidt, U. & Servais, L. Lessons learned from discontinued clinical developments in Duchenne muscular dystrophy. Front. Pharmacol. 12, 735912 (2021).
Markati, T. et al. Emerging therapies for Duchenne muscular dystrophy. Lancet Neurol. 21, 814–829 (2022).
Italfarmaco Group Announces Positive Topline Data From Phase 3 Trial Showing Beneficial Effect Of Givinostat in Patients with Duchenne Muscular Dystrophy (Businesswire, 2022); https://www.businesswire.com/news/home/20220625005001/en/Italfarmaco-Group-Announces-Positive-Topline-Data-from-Phase-3-Trial-Showing-Beneficial-Effect-of-Givinostat-in-Patients-with-Duchenne-Muscular-Dystrophy.
Colussi, C. et al. HDAC2 blockade by nitric oxide and histone deacetylase inhibitors reveals a common target in Duchenne muscular dystrophy treatment. Proc. Natl Acad. Sci. USA 105, 19183–19187 (2008).
Bettica, P. et al. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul. Disord. 26, 643–649 (2016).
Webster, C., Silberstein, L., Hays, A. P. & Blau, H. M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell 52, 503–513 (1988).
Petrof, B. J. et al. Adaptations in myosin heavy chain expression and contractile function in dystrophic mouse diaphragm. Am. J. Physiol. 265, C834–C841 (1993).
Oldfors, A. Hereditary myosin myopathies. Neuromuscul. Disord. 17, 355–367 (2007).
Clinical Trials (Edgewise, accessed 2023); https://edgewisetx.com/clinical-trials.
Cordova, G., Negroni, E., Cabello-Verrugio, C., Mouly, V. & Trollet, C. Combined therapies for Duchenne muscular dystrophy to optimize treatment efficacy. Front. Genet. 9, 114 (2018).
Verhaart, I. E. C. et al. Prednisolone treatment does not interfere with 2’-O-methyl phosphorothioate antisense-mediated exon skipping in Duchenne muscular dystrophy. Hum. Gene Ther. 23, 262–273 (2012).
Peccate, C. et al. Antisense pre-treatment increases gene therapy efficacy in dystrophic muscles. Hum. Mol. Genet. 25, 3555–3563 (2016).
Kendall, G. C. et al. Dantrolene enhances antisense-mediated exon skipping in human and mouse models of Duchenne muscular dystrophy. Sci. Transl. Med. 4, 164ra160 (2012).
Bizot, F. et al. Histone deacetylase inhibitors improve antisense-mediated exon-skipping efficacy in mdx mice. Mol. Ther. Nucleic Acids 30, 606–620 (2022).
Guiraud, S. et al. The potential of utrophin and dystrophin combination therapies for Duchenne muscular dystrophy. Hum. Mol. Genet. 28, 2189–2200 (2019).
Hayashita-Kinoh, H. et al. Improved transduction of canine X-linked muscular dystrophy with rAAV9-microdystrophin via multipotent MSC pretreatment. Mol. Ther. Methods Clin. Dev. 20, 133–141 (2021).
Roberts, T. C. The microRNA machinery. Adv. Exp. Med. Biol. 887, 15–30 (2015).
Greco, S. et al. Common micro-RNA signature in skeletal muscle damage and regeneration induced by Duchenne muscular dystrophy and acute ischemia. FASEB J. 23, 3335–3346 (2009).
Roberts, T. C. et al. Expression analysis in multiple muscle groups and serum reveals complexity in the microRNA transcriptome of the mdx mouse with implications for therapy. Mol. Ther. Nucleic Acids 1, e39 (2012).
Cacchiarelli, D. et al. miR-31 modulates dystrophin expression: new implications for Duchenne muscular dystrophy therapy. EMBO Rep. 12, 136–141 (2011).
Fiorillo, A. A. et al. TNF-α-induced microRNAs control dystrophin expression in becker muscular dystrophy. Cell Rep. 12, 1678–1690 (2015).
Basu, U. et al. Translational regulation of utrophin by miRNAs. PLoS ONE 6, e29376 (2011).
Mishra, M. K., Loro, E., Sengupta, K., Wilton, S. D. & Khurana, T. S. Functional improvement of dystrophic muscle by repression of utrophin: let-7c interaction. PLoS ONE 12, e0182676 (2017).
Abmayr, S., Gregorevic, P., Allen, J. M. & Chamberlain, J. S. Phenotypic improvement of dystrophic muscles by rAAV/microdystrophin vectors is augmented by Igf1 codelivery. Mol. Ther. 12, 441–450 (2005).
Dumonceaux, J. et al. Combination of myostatin pathway interference and dystrophin rescue enhances tetanic and specific force in dystrophic mdx mice. Mol. Ther. 18, 881–887 (2010).
Malerba, A. et al. Dual myostatin and dystrophin exon skipping by morpholino nucleic acid oligomers conjugated to a cell-penetrating peptide is a promising therapeutic strategy for the treatment of Duchenne muscular dystrophy. Mol. Ther. Nucleic Acids 1, e62 (2012).
Rodino-Klapac, L. R. et al. Micro-dystrophin and follistatin co-delivery restores muscle function in aged DMD model. Hum. Mol. Genet. 22, 4929–4937 (2013).
Mariot, V. et al. Downregulation of myostatin pathway in neuromuscular diseases may explain challenges of anti-myostatin therapeutic approaches. Nat. Commun. 8, 1859 (2017).
Godfrey, C. et al. How much dystrophin is enough: the physiological consequences of different levels of dystrophin in the mdx mouse. Hum. Mol. Genet. 24, 4225–4237 (2015).
van den Bergen, J. C. et al. Dystrophin levels and clinical severity in Becker muscular dystrophy patients. J. Neurol. Neurosurg. Psychiatry 85, 747–753 (2014).
Hoffman, E. P. et al. Improved diagnosis of Becker muscular dystrophy by dystrophin testing. Neurology 39, 1011–1017 (1989).
van Westering, T. L. E. et al. Uniform sarcolemmal dystrophin expression is required to prevent extracellular microRNA release and improve dystrophic pathology. J. Cachexia Sarcopenia Muscle 11, 578–593 (2020).
Chwalenia, K. et al. Exon skipping induces uniform dystrophin rescue with dose-dependent restoration of serum miRNA biomarkers and muscle biophysical properties. Mol. Ther. Nucleic Acids 29, 955–968 (2022).
Dangouloff, T. & Servais, L. Clinical evidence supporting early treatment of patients with spinal muscular atrophy: current perspectives. Ther. Clin. Risk Manag. 15, 1153–1161 (2019).
Thomas, S. et al. Time to diagnosis of Duchenne muscular dystrophy remains unchanged: findings from the Muscular Dystrophy Surveillance, Tracking, and Research Network, 2000-2015. Muscle Nerve 66, 193–197 (2022).
Author information
Authors and Affiliations
Contributions
The manuscript was conceived by K.E.D. and T.C.R. The first draft was written by T.C.R. All authors researched data for the article. All authors contributed substantially to discussion of the content and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
K.E.D. is a member of the scientific advisory board of Sarepta Therapeutics. M.J.A.W. is an adviser and shareholder in PepGen Ltd and Evox Therapeutics. T.C.R. declares no financial competing interests.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Alessandra Ferlini, Oxana Ibraghimov-Beskrovnaya and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roberts, T.C., Wood, M.J.A. & Davies, K.E. Therapeutic approaches for Duchenne muscular dystrophy. Nat Rev Drug Discov 22, 917–934 (2023). https://doi.org/10.1038/s41573-023-00775-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-023-00775-6