Abstract
Purpose:
We report on a case in which cell-free fetal DNA was positive for trisomy 13 most likely due to confined placental mosaicism. Cell-free fetal DNA testing analyzes DNA derived from placental trophoblast cells and can lead to incorrect results that are not representative of the fetus.
Methods:
We sought to confirm commercial cell-free fetal DNA testing results by chorionic villus sampling and amniocentesis. These results were followed up by postnatal chromosome analysis of cord blood and placental tissue.
Results:
First-trimester cell-free fetal DNA test results were positive for trisomy 13. Cytogenetic analysis of chorionic villus sampling yielded a mosaic karyotype of 47,XY,+13[10]/46,XY[12]. G-banded analysis of amniotic fluid was normal, 46,XY. Postnatal cytogenetic analysis of cord blood was normal. Karyotyping of tissues from four quadrants of the placenta demonstrated mosaicism for trisomy 13 in two of the quadrants and a normal karyotype in the other two.
Conclusion:
Our case illustrates several important aspects of this new testing methodology: that cell-free fetal DNA may not be representative of the fetal karyotype; that follow-up with diagnostic testing of chorionic villus sampling and/or amniotic fluid for abnormal test results should be performed; and that pretest counseling regarding the full benefits, limitations, and possible testing outcomes of cell-free fetal DNA screening is important.
Genet Med 15 9, 729–732.
Similar content being viewed by others
Main
In late 2011, maternal serum cell-free fetal DNA (cffDNA) testing for Down syndrome became available to pregnant women.1 Testing for trisomy 13 and trisomy 18 were added in early 2012.1 This testing offers a noninvasive option for select aneuploidies by massively parallel shotgun sequencing of cffDNA present in a maternal blood sample.1 By assigning each sequencing read to its respective chromosome, an overrepresentation of DNA fragments from a chromosome can be detected, indicating trisomies, most commonly for chromosomes 13, 18, and 21.1 Reported detection rates from laboratory validation studies are 99–100% and 97–100% for trisomy 21 and 18, respectively, with detection rates more variable for trisomy 13, ranging from 78 to 91%.1,2,3 False-positive rates for all trisomies are <1%.1,2,3
cffDNA present in the maternal blood is thought to originate from apoptosis of cytotrophoblast and syncytiotrophoblast cells.4,5 During early embryogenesis, the blastocyst gives rise to both the trophoblast cells, which form the syncytiotrophoblast and cytotrophoblast cells of the placenta, and the inner cell mass, which forms the extraembryonic mesenchymal core of the chorionic villi, the amnion, and the embryo.6,7 Direct chorionic villus (CV) preps reflect karyotypes derived from trophoblast cells, whereas cultured CV sampling (CVS) karyotypes reflect the mesenchymal core cells of the chorionic villi.6 Cells from amniocentesis are derived from the inner cell mass.6 During the development of these lineages, mitotic nondisjunction or trisomy rescue can result in the presence of both normal and trisomic cell lines.6 Confined placental mosaicism (CPM) for a trisomy occurs when the trisomic cells are confined to either the placental trophoblast or the mesenchymal villus core cells (or both) and the fetal cells are normal 46,XY or XX.8
Here, we report on a case with positive cffDNA testing for trisomy 13, which was confined only to the placenta. It is important for patients and health-care providers to recognize the potential for false-positive aneuploidy fetal test results due to CPM.
Materials and Methods
cffDNA testing
Maternal venous blood was collected by standard techniques in a sodium heparin tube. MaterniT21 Plus cffDNA testing was performed by Sequenom, San Diego, CA.
CV culture
CV tissue was collected transabdominally and sent to the Indiana University Prenatal Cytogenetics Clinical Laboratory. Upon receipt, CVS cultures were established and maintained using standard culture techniques.9 Twenty-two metaphase cells from four separate cultures were analyzed, and two normal and two abnormal cells were captured and karyotyped.
Amniotic fluid cell culture
At 15 + 5 weeks gestation, amniotic fluid was obtained by standard methods, placed in three 15 ml conical tubes, and sent to the Indiana University Prenatal Cytogenetics Clinical Laboratory. Fetal cells from two tubes were cultured and one tube of fluid (uncultured) was used to perform fluorescence in situ hybridization (FISH) (see below). Thirty-two metaphases from 32 colonies were analyzed from four separate cultures. Three normal cells were captured and karyotyped.
FISH
To perform AneuVysion FISH following a standard protocol (Abbott Molecular, Des Plaines, IL), 5 mg of the original CV sample and 5 ml of amniotic fluid were used. One hundred interphase nuclei were scored for each probe set (X, Y, 18 and 13, 21) from the CVS and 295 total nuclei (100 for X, Y, 18 and 195 for 13, 21) were scored from the amniotic fluid.
Results
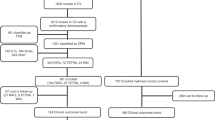
The mother was a 36-year-old, G2P1001 woman who underwent cffDNA testing at 13 + 6 weeks gestation due to advanced maternal age. The test was positive for trisomy 13 with a Z-score of 22.6 (Z-score ≥3 is a positive test) and a fetal cell fraction of 17%. To confirm these results, diagnostic testing by transabdominal CVS was performed at 14 + 3 weeks gestation. Interphase FISH studies, reported at 14 + 5 weeks gestation, showed 61 cells with three chromosome 13 signals and 38 cells with two chromosome 13 signals. The CV sample was cultured and 22 cells were analyzed with a karyotype of 47,XY,+13[10]/46,XY[12], indicating mosaicism for trisomy 13 (see Table 1 ), which was reported out at 15 + 3 weeks gestation.
A follow-up amniocentesis was performed at 15 + 5 weeks gestation. FISH analysis was normal with diploid signals for chromosome 13 in all 195 cells scored and was reported out at 16 + 4 weeks gestation. Chromosome analysis, reported out at 16 + 6 weeks gestation, demonstrated a normal male karyotype, 46,XY, indicating the mosaicism for trisomy 13 was likely confined to the placenta, although fetal trisomy 13 mosaicism could not be excluded.
An extensive ultrasound at 17 + 6 weeks gestation revealed no structural abnormalities or other markers for aneuploidy, and normal growth. Subsequently, serial ultrasounds were performed for reevaluation of the fetal anatomy and assessment of growth. At 23 + 3 weeks gestation, weekly umbilical artery Doppler evaluation and biophysical profiles were initiated. Growth restriction and an elevated umbilical artery systolic to end-diastolic ratio were noted at 23 + 5 weeks gestation. Further increased antenatal testing in the form of nonstress testing was initiated at 32 weeks gestation. The fetus continued to show intrauterine growth restriction with normal umbilical artery Doppler and biophysical profiles evaluations. At 36 + 6 weeks, oligohydramnios with an amniotic fluid index of 4.6 cm (amniotic fluid index of <5 cm is considered oligohydramnios) was noted and a cesarean section was performed.
The newborn’s length, weight, and head circumference were 2,135 g (3rd percentile), 47.5 cm (25th–50th percentile), and 33 cm (50th percentile), respectively. Apgar scores were 7 at 1 min and 9 at 5 min. Both cord blood and placental tissue were collected at delivery. Chromosome analysis of the cord blood yielded 50 cells with a normal karyotype, 46,XY. Tissue from four quadrants of the placenta was also collected. In quadrant A, 43 cells were counted. Five cells showed trisomy 13 and 38 cells were normal, 47,XY,+13[5]/46,XY[38]. In quadrant B, 20 cells were counted. Mosaicism was detected with 5 cells trisomic for 13 and 15 normal cells, 47,XY,+13[5]/46,XY[15]. In quadrants C and D, 50 and 29 cells, respectively, were counted, and all cells were normal, 46,XY. (see Table 1 ). An exam at 1 day of age, and at 83 days of age by a medical geneticist were normal and revealed no physical features of trisomy 13.
Discussion
We report on a case with a positive maternal serum cffDNA test for trisomy 13. Diagnostic testing indicated trisomy 13 mosaicism by FISH and chromosome analyses in cultured CV tissue, normal chromosomes by FISH and karyotype analysis of amniotic fluid cells, and normal chromosomes from postnatal cord blood. We conclude that the cffDNA results were due to mosaicism in the placenta but not the fetus, indicating false-positive results for the fetal karyotype due to CPM.
Mosaicism is defined as the presence of two or more karyotypically distinct cell populations. Mosaicism for a trisomy can arise due to postzygotic mitotic nondisjunction in an embryo that was originally diploid, or postzygotic mitotic trisomy rescue in an embryo that was originally trisomic.10 Depending on when and in what cell lineage these events occur, mosaicism may be found in different tissues at prenatal diagnosis. Mosaicism may be present only in the placenta (CPM), in both the placenta and the fetus (true fetal mosaicism), or in just the fetus.10 In cases involving the placenta, the cytotrophoblast cells, the villus mesenchymal cells, or both cell types can be chromosomally abnormal or mosaic.10
Large, multicenter studies have determined that the frequency of mosaicism in either direct or cultured analysis of CVS tissue to be 1–2%.8,11 When trisomy mosaicism was detected by CVS in these studies, in ~80% of the cases the chromosome abnormalities were confined to the placenta with normal fetal chromosome constitution. Furthermore, these studies indicated that if the chromosome abnormality were detected by the direct method and therefore confined to the cytotrophoblast tissue and not detected in the cultured chorionic villi, then the chromosome abnormality was never confirmed in fetal tissues (i.e., amniocentesis, cord blood). On the other hand, chromosome abnormalities detected in the cultured chorionic villi were more likely to be confirmed in the fetus. Differences between the fetal and the cytotrophoblast tissues are more common than differences between the fetal and villus mesenchymal tissues, probably because the cytotrophoblasts diverge from the embryo cell lineage earlier than the villus mesenchymal cells. Given that cffDNA tests evaluate DNA fragments believed to be derived from cytotrophoblast cells, this test may only approach true detection rates for aneuploidy of direct CVS as opposed to cultured CVS karyotype analysis. It is worth noting that most cytogenetics laboratories have abandoned direct CVS karyotype analysis due to its increased rate of false-positive and false-negative results.12
Currently, the frequency of false-positive fetal test results with maternal serum cffDNA testing due to CPM or other fetal–placental discrepancy is not known. False-negative results could also arise with cffDNA testing if a trisomic fetus undergoes trisomy rescue only in the placenta. There is more selective pressure on the fetus and therefore this event is unlikely; however, there are rare reports of this event occurring.13 Therefore, it is important that patients receive pretest counseling and give informed consent prior to cffDNA screening. Patients should be made aware of the potential for false positives and false-negative results, and nonspecific results due to differences between the fetus and the placentally derived sample analyzed. Furthermore, it is also critical that all patients with positive maternal serum cffDNA results have cytogenetic confirmation through CVS and/or amniocentesis before making decisions regarding pregnancy termination.
Trisomy 13 occurs with an estimated incidence of 1/8,000 to 1/12,000 live-born infants and 15/10,000 of first-trimester fetuses.14 Trisomy 13 mosaicism is even more rare, given that ~5% of live-born children with trisomy 13 are mosaic.15 Data concerning the outcome of cases with a prenatal diagnosis of trisomy 13 mosaicism are very limited. In the cases of mosaicism detected by CVS, even a normal amniocentesis karyotype cannot rule out the possibility of true fetal mosaicism.15,16 In addition, in cases in which trisomy 13 mosaicism is confirmed from amniocentesis, the outcome may be extremely variable and may range from normal to a characteristic trisomy 13 phenotype.17,18 Complex and difficult counseling situations surrounding a prenatal diagnosis of mosaic trisomy 13 therefore arise. It is important to report such cases in the literature so that more information may be collected and made available to couples for counseling purposes.
A diagnosis of a mosaic trisomy from a CVS may also be associated with the presence of uniparental disomy due to trisomy rescue and have clinical significance if the chromosome involved contains imprinted genes (chromosomes 7, 11, 14, 15, and 16).19 Prenatal uniparental disomy testing is available and should be offered in these situations. In addition, further research may be warranted to determine the optimal management of pregnancies with CPM. Studies have found that pregnancies with CPM are at an increased risk for prenatal and/or perinatal complications such as intrauterine growth restriction. In these situations, therefore, close monitoring of these pregnancies will probably be necessary.20
Previously, CPM was identified only in women who underwent CVS early in pregnancy and was undetected if only maternal serum testing or amniocentesis were performed. Given that cffDNA testing is a noninvasive testing method with high detection rates, it is likely that in the future more pregnant women will undergo this testing and that more cases of CPM will be detected. Therefore, it is important that health-care providers become more familiar with the complexities of these situations.
As with any new test, issues surrounding how this test should be integrated into the clinical setting arise. cffDNA testing is not a simple blood test and needs informed consent before testing. Individuals undergoing this screening should understand all of the potential results. With respect to CVS, individuals should clearly understand the possibility of a false-positive result due to CPM. Because this test analyzes DNA fragments derived from placental tissue, individuals should also understand that this test is not a diagnostic test and that all positive results should be confirmed by a CVS and/or amniocentesis, especially before any further pregnancy management decisions. Explaining these complex results and conveying the uncertainties involved in the testing can be difficult and may create anxiety for patients. Moving forward, more data need to be collected regarding rare events such as mosaicism so that patients can be better informed regarding the impact of abnormal prenatal testing on their pregnancy.
Disclosure
The authors declare no conflict of interest.
References
Palomaki GE, Deciu C, Kloza EM, et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: an international collaborative study. Genet Med 2012;14:296–305.
Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP ; MatErnal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol 2012;119:890–901.
Ashoor G, Syngelaki A, Wagner M, Birdir C, Nicolaides KH . Chromosome-selective sequencing of maternal plasma cell-free DNA for first-trimester detection of trisomy 21 and trisomy 18. Am J Obstet Gynecol 2012;206:322.e1–322.e5.
Flori E, Doray B, Gautier E, et al. Circulating cell-free fetal DNA in maternal serum appears to originate from cyto- and syncytio-trophoblastic cells. Case report. Hum Reprod 2004;19:723–724.
Tjoa ML, Cindrova-Davies T, Spasic-Boskovic O, Bianchi DW, Burton GJ . Trophoblastic oxidative stress and the release of cell-free feto-placental DNA. Am J Pathol 2006;169:400–404.
Kalousek DK, Vekemans M . Confined placental mosaicism. J Med Genet 1996;33:529–533.
Markert CL, Petters RM . Manufactured hexaparental mice show that adults are derived from three embyronic cells. Science 1978;202:56–58.
Hahnemann JM, Vejerslev LO . European collaborative research on mosaicism in CVS (EUCROMIC)–fetal and extrafetal cell lineages in 192 gestations with CVS mosaicism involving single autosomal trisomy. Am J Med Genet 1997;70:179–187.
Barch MJ, Knutsen T, Spurbeck J . The AGT Cytogenetics Laboratory Manual, 3rd edn, Lippincott-Raven Publishers: Philadelphia, 1997.
Kalousek DK, Dill FJ . Chromosomal mosaicism confined to the placenta in human conceptions. Science 1983;221:665–667.
Ledbetter DH, Zachary JM, Simpson JL, et al. Cytogenetic results from the U.S. Collaborative Study on CVS. Prenat Diagn 1992;12:317–345.
Phillips OP, Velagaleti GV, Tharapel AT, Shulman LP . Discordant direct and culture results following chorionic villus sampling and the diagnosis of a third cell line in the fetus. Prenat Diagn 1997;17:170–172.
Fischer W, Dermitzel A, Osmers R, Pruggmayer M . Complete karyotype discrepancy between placental and fetal cells in a case of ring chromosome 18. Prenat Diagn 2001;21:481–483.
Snijders RJ, Holzgreve W, Cuckle H, Nicolaides KH . Maternal age-specific risks for trisomies at 9-14 weeks’ gestation. Prenat Diagn 1994;14:543–552.
Chen M, Yeh GP, Shih JC, Wang BT . Trisomy 13 mosaicism: study of serial cytogenetic changes in a case from early pregnancy to infancy. Prenat Diagn 2004;24:137–143.
Stetten G, Escallon CS, South ST, McMichael JL, Saul DO, Blakemore KJ . Reevaluating confined placental mosaicism. Am J Med Genet A 2004;131:232–239.
Delatycki MB, Pertile MD, Gardner RJ . Trisomy 13 mosaicism at prenatal diagnosis: dilemmas in interpretation. Prenat Diagn 1998;18:45–50.
Griffith CB, Vance GH, Weaver DD . Phenotypic variability in trisomy 13 mosaicism: two new patients and literature review. Am J Med Genet A 2009;149A:1346–1358.
Gardner RJM, Sutherland GR . Chromosome Abnormality and Genetic Counseling, 3rd edn. Oxford University Press: New York, 2004.
Baffero GM, Somigliana E, Crovetto F, et al. Confined placental mosaicism at chorionic villous sampling: risk factors and pregnancy outcome. Prenat Diagn 2012;32:1102–1108.
Acknowledgements
The authors thank the family for their willingness to participate in the publication of their case. We also thank Janelle Strobel, Jennifer Whitcomb, Shawn Lancaster, and Patrick Hanlin for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hall, A., Drendel, H., Verbrugge, J. et al. Positive cell-free fetal DNA testing for trisomy 13 reveals confined placental mosaicism. Genet Med 15, 729–732 (2013). https://doi.org/10.1038/gim.2013.26
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2013.26
Keywords
This article is cited by
-
Efficiency of non-invasive prenatal screening in pregnant women at advanced maternal age
BMC Pregnancy and Childbirth (2021)
-
Next-generation sequencing: a follow-up of 36,913 singleton pregnancies with noninvasive prenatal testing in central China
Journal of Assisted Reproduction and Genetics (2020)
-
Clinical application of noninvasive prenatal screening for sex chromosome aneuploidies in 50,301 pregnancies: initial experience in a Chinese hospital
Scientific Reports (2019)
-
Analysis of the accuracy of Z-scores of non-invasive prenatal testing for fetal Trisomies 13, 18, and 21 that employs the ion proton semiconductor sequencing platform
Molecular Cytogenetics (2018)
-
Performance of non-invasive prenatal testing for trisomies 21 and 18 in twin pregnancies
Molecular Cytogenetics (2018)