Abstract
Background/objectives:
To investigate the relationship between postprandial nutrient balance, satiety and hormone changes induced by two different meals taken after a moderate intensity exercise bout.
Subjects/Methods:
Ten prepubertal obese children participated in the study. The experiment was designed as a cross-over study for repeated measures. Each test period lasted five consecutive hours during which the children were under medical supervision. The effects of two isocaloric meals were compared after a moderate intensity exercise (4 multiples of resting metabolic rate, 30 min, cycling): a low-fat/high-carbohydrate meal (meal A) and a high-fat/low-carbohydrate meal (meal B). Pre and postprandial (3 h) substrate oxidation, biochemical parameters, gastrointestinal hormone concentrations and appetite were measured.
Results:
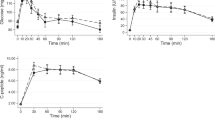
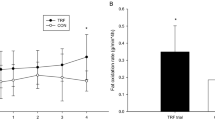
The main results were: (i) higher fat balance (5.1±5.0 vs −5.0±6.6 g, P=0.001) and lower carbohydrate balance after meal B than A (−9.7±13.3 vs 11.3±18.3 g, P<0.01); (ii) higher energy balance after meal B than after meal A (5.9±21.5 vs −13.9±20.2 kcal, P<0.05); (iii) higher plasma triglyceride concentrations (area under the curve) after meal B than after meal A (2962.5±2095.8 mg*180 min/dl vs −169.5±1633.7 mg*180 min/dl, P<0.01); (iv) higher serum glucagon-like peptide-1 concentrations after meal B than after meal A (1101.5±873.0 pmol*180 min/l vs 478.8±638.3 pmol*180 min/l, P<0.05).
Conclusions:
After a bout of moderate intensity exercise, a meal with a high-fat/low-carbohydrate ratio had a less favorable metabolic impact than an isoenergetic, isoproteic low-fat/high-carbohydrate meal.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nemet D, Barkan S, Epstein Y, Friedland O, Kowen G, Eliakim A . Short- and long-term beneficial effects of a combined dietary-behavioral-physical activity intervention for the treatment of childhood obesity. Pediatrics 2005; 115: e443–e449.
Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O’Malley C, Stolk RP et al. Interventions for treating obesity in children. Cochrane Database Syst Rev 2009; 21: CD001872.
King NA, Blundell JE . High-fat foods overcome the energy expenditure induced by high-intensity cycling or running. Eur J Clin Nutr 1995; 49: 114–123.
Pillard F, Van Wymelbeke V, Garrigue E, Moro C, Crampes F, Guilland JC et al. Lipid oxidation in overweight men after exercise and food intake. Metabolism 2010; 59: 267–274.
Martins C, Morgan LM, Bloom SR, Robertson MD . Effects of exercise on gut peptides, energy intake and appetite. J.Endocrinol 2007; 193: 251–258.
Onaga T, Zabielski R, Kato S . Multiple regulation of peptide YY secretion in the digestive tract. Peptides 2002; 23: 279–290.
Murphy KG, Bloom SR . Gut hormones and the regulation of energy homeostasis. Nature 2006; 444: 854–859.
Tanner JM, Whitehouse RH . Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child 1976; 51: 170–179.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH . Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320: 1240–1243.
Luciano A, Bressan F, Zoppi G . Body mass index reference curves for children aged 3-19 years from Verona, Italy. Eur J Clin Nutr 1997; 51: 6–10.
Maffeis C, Provera S, Filippi L, Sidoti G, Schena S, Pinelli L et al. Distribution of food intake as a risk factor for childhood obesity. Int J Obes 2000; 24: 75–80.
Bandini LG, Schoeller DA, Dietz WH . Energy expenditure in obese and nonobese adolescents. Pediatr Res 1990; 27: 198–203.
Maffeis C, Armellini F, Tatò L, Schutz Y . Fat oxidation and adiposity in prepubertal children: exogenous versus endogenous fat utilization. J Clin Endocrinol Metab 1999; 84: 654–658.
Flint A, Raben A, Blundell JE, Reproducibility Astrup A . power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 2000; 24: 38–48.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Weir JDV . New methods of calculating metabolic rate with special reference to protein metabolism. J Phisiol 1949; 109: 1–9.
Jequier E, Felber JP . Indirect calorimetry. In: KGMM Alberti, Home PD, Taylor R, (eds). Clinical Endocrinology and Metabolism. Bailliere Tindall: London, 1987; 911–935.
Davis CL, Pollock NK, Waller JL, Allison JD, Dennis BA, Bassali R, Meléndez A, Boyle CA, Gower BA . Exercise dose and diabetes risk in overweight and obese children: a randomized controlled trial. JAMA 2012; 308: 1103–1112.
Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S . Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes 2012; 61: 2787–2795.
Rijkelijkhuizen JM, McQuarrie K, Girman CJ, Stein PP, Mari A, Holst JJ, Nijpels G, Dekker JM . Effects of meal size and composition on incretin, alpha-cell, and beta-cell responses. Metabolism 2010; 59: 502–511.
Maffeis C, Surano MG, Cordioli S, Gasperotti S, Corradi M, Pinelli L . A high-fat vs a moderate-fat meal in obese boys: nutrient balance, appetite, and gastrointestinal hormone changes. Obesity 2010; 18: 449–455.
Treuth MS, Sunehag AL, Trautwein LM, Bier DM, Haymond MW, Butte NF . Metabolic adaptation to high-fat and high-carbohydrate diets in children and adolescents. Am J Clin Nutr 2003; 77: 479–489.
Bayturan O, Tuzcu EM, Lavoie A, Hu T, Wolski K, Schoenhagen P et al. The metabolic syndrome, its component risk factors, and progression of coronary atherosclerosis. Arch Intern Med 2010; 170: 478–484.
Ooi EM, Barrett PH, Chan DC, Watts GF . Apolipoprotein C-III: understanding an emerging cardiovascular risk factor. Clin Sci 2008; 114: 611–624.
Zheng C, Khoo C, Furtado J, Sacks FM . Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense LDL phenotype. Circulation 2010; 121: 1722–1734.
Kamagate A, Dong HH . FoxO1 integrates insulin signaling to VLDL production. Cell Cycle 2008; 7: 3162–3170.
Ramos-Roman MA, Lapidot SA, Phair RD, Parks EJ . Insulin activation of plasma non-esterified fatty acid uptake in metabolic syndrome. Arterioscler Thromb Vasc Biol 2012; 32: 1799–1808.
Drucker DJ, Nauck MA . The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705.
Mingrone G, Nolfe G, Gissey GC, Iaconelli A, Leccesi L, Guidone C et al. Circadian rhythms of GIP and GLP1 in glucose-tolerant and in type 2 diabetic patients after biliopancreatic diversion. Diabetologia 2009; 52: 873–881.
Flint A, Raben A, Astrup A, Holst JJ . Glucagon-like peptide 1promotes satiety and suppresses energy intake in humans. J Clin Invest 1998; 101: 515–520.
Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol 1999; 276: R1541–R1544.
Zander M, Madsbad S, Madsen JL, Holst JJ . Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002; 359: 824–830.
Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A . The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes 2001; 25: 781–792.
Shalev A, Holst JJ, Keller U . Effects of glucagon-like peptide 1 (7-36 amide) on whole-body protein metabolism in healthy man. Eur J Clin Invest 1997; 27: 10–16.
Flint A, Kapitza C, Hindsberger C, Zdravkovic M . The once daily human glucagon-like peptide-1 (GLP-1) analog liraglutide improves postprandial glucose levels in type 2 diabetes patients. Adv Ther 2011; 28: 213–226.
Ratner RE, Maggs D, Nielsen LL, Stonehouse AH, Poon T, Zhang B et al. Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus. Diabetes Obes Metab 2006; 8: 419–428.
Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebocontrolled study. Lancet 2009; 374: 1606–1616.
Acknowledgements
We thank Marta Bianchi and Camilla Melegari for their critical revision of the manuscript. The work was supported by the Ministry of Health, Research Project of National Interest (PRIN) No. 2008CJ7CTW, by a grant of Barilla SpA, Parma (Italy) and by funds from the University of Verona.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Maffeis, C., Bonadonna, R., Maschio, M. et al. Metabolic and hormonal consequences of two different meals after a moderate intensity exercise bout in obese prepubertal children. Eur J Clin Nutr 67, 725–731 (2013). https://doi.org/10.1038/ejcn.2013.86
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.86