Abstract
Background/Objectives:
The goal of this work is to estimate the reduction in mortality rates for six geopolitical regions of the world under the assumption that serum 25-hydroxyvitamin D (25(OH)D) levels increase from 54 to 110 nmol/l.
Subjects/Methods:
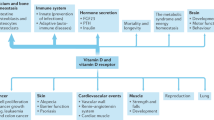
This study is based on interpretation of the journal literature relating to the effects of solar ultraviolet-B (UVB) and vitamin D in reducing the risk of disease and estimates of the serum 25(OH)D level–disease risk relations for cancer, cardiovascular disease (CVD) and respiratory infections. The vitamin D-sensitive diseases that account for more than half of global mortality rates are CVD, cancer, respiratory infections, respiratory diseases, tuberculosis and diabetes mellitus. Additional vitamin D-sensitive diseases and conditions that account for 2 to 3% of global mortality rates are Alzheimer's disease, falls, meningitis, Parkinson's disease, maternal sepsis, maternal hypertension (pre-eclampsia) and multiple sclerosis. Increasing serum 25(OH)D levels from 54 to 110 nmol/l would reduce the vitamin D-sensitive disease mortality rate by an estimated 20%.
Results:
The reduction in all-cause mortality rates range from 7.6% for African females to 17.3% for European females. Reductions for males average 0.6% lower than for females. The estimated increase in life expectancy is 2 years for all six regions.
Conclusions:
Increasing serum 25(OH)D levels is the most cost-effective way to reduce global mortality rates, as the cost of vitamin D is very low and there are few adverse effects from oral intake and/or frequent moderate UVB irradiance with sufficient body surface area exposed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ascherio A, Munger KL, Simon KC (2010). Vitamin D and multiple sclerosis. Lancet Neurol 9, 599–612.
Bikle DD (2011). Vitamin D: an ancient hormone. Exp Dermatol 20, 7–13.
Black PN, Scragg R (2005). Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination survey. Chest 128, 3792–3798.
Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM (2007). Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab 92, 3517–3522.
Boscoe FP, Schymura MJ (2006). Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2000. BMC Cancer 6, 264.
Cannell JJ, Hollis BW (2008). Use of vitamin D in clinical practice. Altern Med Rev 13, 6–20.
Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S et al. (2006). Epidemic influenza and vitamin D. Epidemiol Infect 134, 1129–1140.
Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC et al. (2008). Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med 149, 242–250.
Chen W, Clements M, Rahman B, Zhang S, Qiao Y, Armstrong BK (2010). Relationship between cancer mortality/incidence and ambient ultraviolet B irradiance in China. Cancer Causes Control 21, 1701–1709.
Davies P (2010). Vitamin D and tuberculosis. Am J Respir Crit Care Med 181, 94, author reply 95.
Devesa SS, Grauman DJ, Blot WJ, Pennello GA, Hoover RN, Fraumeni Jr JF (1999). Atlas of cancer mortality in the United States, 1950–1994. NIH Publication No. 99-4564. http://www3.cancer.gov/atlasplus/new.html(accessed 7 July 2010).
Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ et al. (2011). Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer 128, 1414–1424.
Garland CF, French CB, Baggerly LL, Heaney RP (2011). Vitamin D supplement doses and serum 25-hydroxyvitamin D in the range associated with cancer prevention. Anticancer Res 31, 617–622.
Garland CF, Garland FC (1980). Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol 9, 227–231.
Garland CF, Gorham ED, Mohr SB, Garland FC (2009). Vitamin D for cancer prevention: global perspective. Ann Epi 19, 468–483.
Garland FC, Garland CF, Gorham ED, Young JF (1990). Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med 19, 614–622.
Gilbert CR, Arum SM, Smith CM (2009). Vitamin D deficiency and chronic lung disease. Can Respir J 16, 75–80.
Gillie O (2010). Sunlight robbery: a critique of public health policy on vitamin D in the UK. Mol Nutr Food Res 54, 1148–1163.
Gombart AF (2009). The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol 4, 1151–1165.
Grant WB (2002). An estimate of premature cancer mortality in the US due to inadequate doses of solar ultraviolet-B radiation. Cancer 94, 1867–1875.
Grant WB (2007a). An ecologic study of cancer mortality rates in Spain with respect to indices of solar UV irradiance and smoking. Int J Cancer 120, 1123–1127.
Grant WB (2007b). A meta-analysis of second cancers after a diagnosis of nonmelanoma skin cancer: additional evidence that solar ultraviolet-B irradiance reduces the risk of internal cancers. J Steroid Biochem Mol 103, 668–674.
Grant WB (2009a). Does vitamin D reduce the risk of dementia? J Alzheimers Dis 17, 151–149.
Grant WB (2009b). In defense of the sun: an estimate of changes in mortality rates in the United States if mean serum 25-hydroxyvitamin D levels were raised to 45 ng/mL by solar ultraviolet-B irradiance. Dermato-Endocrinology 1, 207–214.
Grant WB (2009c). How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer? An examination using Hill's criteria for causality. Dermato-Endocrinology 1, 17–24.
Grant WB (2009d). A critical review of Vitamin D and cancer: a report of the IARC Working Group on vitamin D. Dermato-Endocrinology 1, 25–33.
Grant WB (2009e). Solar ultraviolet-B irradiance and vitamin D may reduce the risk of septicemia. Dermato-Endocrinology 1, 37–42.
Grant WB (2009f). Critique of the U-shaped serum 25-hydroxyvitamin D level-disease response relation. Dermato-Endocrinology 1, 289–293.
Grant WB (2010a). An ecological study of cancer incidence and mortality rates in France with respect to latitude, an index for vitamin D production. Deramato-Endocrinology 2, 62–67.
Grant WB (2010b). Relation between prediagnostic serum 25-hydroxyvitamin D level and incidence of breast, colorectal, and other cancers. J Photochem Photobiol B 101, 130–136.
Grant WB (2010c). An ecological study of cancer mortality rates in the United States with respect to solar ultraviolet-B doses, smoking, alcohol consumption, and urban/rural residence. Deramato-Endocrinology 2, 68–76.
Grant WB (2010d). The prevalence of multiple sclerosis in 3 US communities: the role of vitamin D (letter). Prev Chronic Dis 7, A89.
Grant WB (2011a). Effect of interval between serum draw and follow-up period on relative risk of cancer incidence with respect to 25-hydroxyvitamin D level; implications for meta-analyses and setting vitamin D guidelines. Dermato-Endocrinology 3(3); e-pub ahead of print July/August/September 2011.
Grant WB (2011b). Is the Institute of Medicine report on calcium and vitamin D good science? Biol Res Nurs 13, 117–119.
Grant WB, Boucher BJ (2010). Are Hill's criteria for causality satisfied for vitamin D and periodontal disease? Dermato-Endocrinology 2, 30–36.
Grant WB, Boucher BJ (2011). Requirements for vitamin D across the lifespan. Biol Res Nurs 13, 120–133.
Grant WB, Cross HS, Garland CF, Gorham ED, Moan J, Peterlik M et al. (2009). Estimated benefit of increased vitamin D status in reducing the economic burden of disease in Western Europe. Prog Biophys Mol Biol 99, 104–113.
Grant WB, Garland CF (2004). A critical review of studies on vitamin D in relation to colorectal cancer. Nutr Cancer 48, 115–123.
Grant WB, Garland CF (2006). The association of solar ultraviolet B (UVB) with reducing risk of cancer: multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res 26, 2687–2699.
Grant WB, Giovannucci E (2009). The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918–1919 influenza pandemic in the United States. Dermato-Endocrinology 1, 215–219.
Grant WB, Juzeniene A, Moan JE (2011). Health benefit of increased serum 25(OH)D levels from oral intake and ultraviolet-B irradiance in the Nordic countries. Scand J Public Health 39, 70–78.
Grant WB, Mohr SB (2009). Ecological studies of ultraviolet B, vitamin D and cancer since 2000. Ann Epidemiol 19, 446–454.
Grant WB, Peiris AN (2010). Possible role of serum 25-hydroxyvitamin D in Black-White health disparities in the United States. J Am Med Directors Assoc 11, 617–628.
Grant WB, Schuitemaker G (2010). Health benefits of higher serum 25-hydroxyvitamin D levels in The Netherlands. J Steroid Biochem Molec Biol 121, 456–458.
Grant WB, Schwalfenberg GK, Genuis SJ, Whiting SJ (2010). An estimate of the economic burden and premature deaths due to vitamin D deficiency in Canada. Molec Nutr Food Res 54, 1127–1133.
Guillot X, Semerano L, Saidenberg-Kermanac’h N, Falgarone G, Boissier MC (2010). Vitamin D and inflammation. Joint Bone Spine 77, 552–557.
Hagenau T, Vest R, Gissel TN, Poulsen CS, Erlandsen M, Mosekilde L et al. (2009). Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int 20, 133–140.
Hanwell HE, Banwell B (2011). Assessment of evidence for a protective role of vitamin D in multiple sclerosis. Biochim Biophys Acta 1812, 202–212.
Heaney RP, Holick MF (2011). Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res 26, 455–457.
Helzlsouer KJ, (2010). Overview of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 172, 4–9.
Hill AB (1965). The environment and disease: association or causation? Proc R Soc Med 58, 295–300.
Holick MF (2011). The D-batable institute of medicine report: a D-lightful perspective. Endocr Pract 17, 143–149.
Holick MF (2007). Vitamin D deficiency. N Engl J Med 357, 266–281.
Ho-Pham LT, Nguyen ND, Nguyen TT, Nguyen DH, Bui PK, Nguyen VN et al. (2010). Association between vitamin D insufficiency and tuberculosis in a Vietnamese population. BMC Infect Dis 10, 306.
IARC (2008). Working Group Report 5: Vitamin D and Cancer. IARC: Lyon, France.
Jablonski NG, Chaplin G (2010). Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci USA 107 (Suppl 2), 8962–8968.
Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE et al. (2006). Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 354, 669–683.
Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I et al. (2010). Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 65, 215–220.
Kenborg L, Lassen CF, Ritz B, Schernhammer ES, Hansen J, Gatto NM et al. (2011). Outdoor work and risk for Parkinson's disease: a population-based case-control study. Occup Environ Med 68, 273–278.
Kinlin LM, Spain CV, Ng V, Johnson CC, White AN, Fisman DN (2009). Environmental exposures and invasive meningococcal disease: an evaluation of effects on varying time scales. Am J Epidemiol 169, 588–595.
LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q et al. (2005). Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis 45, 1026–1033.
Lappe JM, Davies KM, Travers-Gustafson D, Heaney RP (2006). Vitamin D status in a rural postmenopausal female population. J Am Coll Nutr 25, 395–402.
Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP (2007). Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 85, 1586–1591.
Leffell DJ, Brash DE (1996). Sunlight and skin cancer. Sci Am 275, 52–53, 56-59.http://toms.gsfc.nasa.gov/ery_uv/dna_exp.gif (accessed 7 July 2010).
Lefkowitz ES, Garland CF (1994). Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int J Epidemiol 23, 1133–1136.
Liu PT, Stenger S, Tang DH, Modlin RL (2007). Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol 179, 2060–2063.
Llewellyn DJ, Lang IA, Langa KM, Melzer D (2011). Vitamin D and cognitive impairment in the elderly US population. J Gerontol A Biol Sci Med Sci 66, 59–65.
Manaseki-Holland S, Qader G, Isaq Masher M, Bruce J, Zulf Mughal M, Chandramohan D et al. (2010). Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health 15, 1148–1155.
Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP et al. (2011). High-dose vitamin D during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet 377, 242–250.
Mizoue T (2004). Ecological study of solar radiation and cancer mortality in Japan. Health Phys 87, 532–538.
Moan J, Lagunova Z, Cicarma E, Aksnes L, Dahlback A, Grant WB et al. (2009). Sunbeds as vitamin D sources. Photochem Photobiol 85, 1474–1479.
Mocanu V, Stitt PA, Costan AR, Voroniuc O, Zbranca E, Luca V et al. (2009). Long-term effects of giving nursing home residents bread fortified with 125 microg (5000 IU) vitamin D per daily serving. Am J Clin Nutr 89, 1132–1137.
Mohr SB (2009). A brief history of vitamin D and cancer prevention. Ann Epidemiol 19, 79–83.
Mookherjee N, Rehaume LM, Hancock RE (2007). Cathelicidins and functional analogues as antisepsis molecules. Expert Opin Ther Targets 11, 993–1004.
Newmark HL, Newmark J (2007). Vitamin D and Parkinson's disease--a hypothesis. Mov Disord 22, 461–468.
Norman AW (2008). From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr 88, 491S–499S.
Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala NB et al. (2010). Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas 65, 225–236.
Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H (2009). Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int 20, 315–322.
Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE et al. (2006). Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 29, 650–656.
Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB (2010). Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care 33, 2021–2023.
Riancho JA, Gonzalez Macias J, Del Arco C, Amado JA, Freijanes J, Anton MA (1987). Vertebral compression fractures and mineral metabolism in chronic obstructive lung disease. Thorax 42, 962–966.
Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD (2010). Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol 203, 366.e1–366.e6.
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK et al. (2011). The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96, 53–58.
Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML (2010). Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One 5, e11088.
Schwartz GG, Hulka BS (1990). Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis). Anticancer Res 10, 1307–1311.
Semba RD, Houston DK, Bandinelli S, Sun K, Cherubini A, Cappola AR et al. (2010). Relationship of 25-hydroxyvitamin D with all-cause and cardiovascular disease mortality in older community-dwelling adults. Eur J Clin Nutr 64, 203–209.
Shanafelt TD, Drake MT, Maurer MJ, Allmer C, Rabe KG, Slager SL et al. (2011). Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia (CLL). Blood 117, 1492–1498.
Sharip A, Sorvillo F, Redelings MD, Mascola L, Wise M, Nguyen DM (2006). Population-based analysis of meningococcal disease mortality in the United States: 1990–2002. Pediatr Infect Dis J 25, 191–194.
Souberbielle JC, Body JJ, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ et al. (2010). Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: recommendations for clinical practice. Autoimmun Rev 9, 709–715.
Toriola AT, Surcel HM, Calypse A, Grankvist K, Luostarinen T, Lukanova A et al. (2010). Independent and joint effects of serum 25-hydroxyvitamin D and calcium on ovarian cancer risk: a prospective nested case-control study. Eur J Cancer 46, 2799–2805.
Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H (2010). Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 91, 1255–1260.
Vaidya A, Forman JP (2010). Vitamin D and hypertension: current evidence and future directions. Hypertension 56, 774–779.
Webb AR, Engelsen O (2006). Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol 82, 1697–1703.
Whiting SJ, Green TJ, Calvo MS (2007). Vitamin D intakes in North America and Asia-Pacific countries are not sufficient to prevent vitamin D insufficiency. J Steroid Biochem Mol Biol 103, 626–630.
Williams B, Williams AJ, Anderson ST (2008). Vitamin D deficiency and insufficiency in children with tuberculosis. Pediatr Infect Dis J 27, 941–942.
World Health Organization (2007). Influenza (seasonal). Fact sheet no 211. Available from: www.who.int/mediacentre/factsheets/fs211/en/print.html.
World Health Organization (2008). The Global Burden of Disease: 2004 Update. GBD 2004 Summary Tables. Health Statistics and Informatics Department, World Health Organization: Geneva, Switzerland. http://www.who.int/healthinfo/global_burden_disease/DTH6%202004.xls.
Yamshchikov AV, Kurbatova EV, Kumari M, Blumberg HM, Ziegler TR, Ray SM et al. (2010). Vitamin D status and antimicrobial peptide cathelicidin (LL-37) concentrations in patients with active pulmonary tuberculosis. Am J Clin Nutr 92, 603–611.
Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H (2010). Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer 46, 2196–2205.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
WB Grant receives or has received funding from the UV Foundation (McLean, VA, USA), the Sunlight Research Forum (Veldhoven, The Netherlands), Bio-Tech-Pharmacal (Fayetteville, AR, USA), the Vitamin D Council (San Luis Obispo, CA, USA) and the Danish Sunbed Federation (Middelfart, Denmark).
Rights and permissions
About this article
Cite this article
Grant, W. An estimate of the global reduction in mortality rates through doubling vitamin D levels. Eur J Clin Nutr 65, 1016–1026 (2011). https://doi.org/10.1038/ejcn.2011.68
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2011.68
Keywords
This article is cited by
-
Targeted 25-hydroxyvitamin D concentration measurements and vitamin D3 supplementation can have important patient and public health benefits
European Journal of Clinical Nutrition (2020)
-
Vitamin D and health in the Mediterranean countries
Hormones (2019)
-
Sinn und Unsinn von Supplementierung in der Onkologie
gynäkologie + geburtshilfe (2019)
-
The importance of vitamin D in maternal and child health: a global perspective
Public Health Reviews (2017)
-
Vitamin D and L-cysteine levels correlate positively with GSH and negatively with insulin resistance levels in the blood of type 2 diabetic patients
European Journal of Clinical Nutrition (2014)