Abstract
Design
Randomised controlled trial
Intervention
Fifty patients (46 females, four males) aged 61.18 ± 12.27 years with a clinical history of continuous symptoms of oral burning or pain for more than six months, no clinical abnormalities that could account for the symptoms and normal blood test findings were allocated using computer randomisation to either the control group who were given information only, or to the experimental group that received the same information plus the tongue protector. The protector consisted of a transparent, low density polyethylene sheath covering the tongue from the tip to the posterior third. The trial length was two months.
Outcome measure
The primary outcome was oral symptoms as recorded using a visual analog scale (VAS) consisting of a 10cm vertical line marked from 0 (=no pain) to 10 (= most severe pain experienced). Secondary outcome was quality of life, measured using OHIP-49 and SF-36.
Results
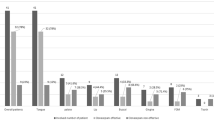
Change in VAS was reported as mean (± s.d.). For the non-intervention group this was 1.4 (± 1.6). For the intervention group it was 3.6 (± 2.2). This difference was statistically significant (P<0.001). For the OHIP-49 the mean changes were 1.92 (± 4.93) and 18.44 (± 29.53) respectively, which was statistically significant (P=0.008). Three of the eight measures in the SF-36 showed a statistically significant improvement in the tongue protector group. Confidence intervals were not reported for any of the measures.
Conclusions
Although a statistical significance was observed in the VAS, studies are needed to reproduce these findings in larger series, over longer periods of time and involving an adequate sample of patients.
Similar content being viewed by others
Commentary
Management of burning mouth syndrome (BMS) is often challenging and sound studies assessing interventions are welcome. BMS is defined as a chronic idiopathic burning discomfort or pain affecting patients with clinically normal, healthy oral mucosa, in whom a medical or dental cause has been excluded.1 Altered taste and symptomatic oral dryness may also be associated with BMS2and as reported in the present study it may cause a significant reduction in affected patient's quality of life.
Historically the quality of evidence relating to interventions for BMS has been limited by a number of factors. Aside from suboptimal study designs, numerous terms have been used to describe the condition including stomatodynia, stomatopyrosis and oral dysaesthesia, with resultant confusion in the literature. Additionally many studies assessing interventions for BMS have included patients with the symptom of burning mouth caused by local and systemic factors (including denture trauma, mucosal pathology, hypersensitivity, haematinic and hormonal deficiencies). The latter can be confusingly referred to as ‘secondary’ BMS but such factors should be excluded prior to making a diagnosis of BMS, as has occurred in the present study.
The aetiology of BMS remains unclear but studies increasingly suggest an underlying neuropathic origin which may be influenced by or result in psychological conditions.2 A small cross-sectional observational study undertaken by this group3 found a high proportion of the BMS patients studied (39/60) had oral parafunctional habits (bruxism, tongue thrusting and biting and lip biting). Such patients might be strictly described as having burning mouth rather than BMS, however, this suggested the possibility that tongue parafunctional habits might cause and/or perpetuate neuropathic changes in the tongue culminating in BMS.
In this prospective, randomised study, the impact of BMS information, advice to avoid parafunctional habits with a behavior modifying technique in one group was compared with the same advice bundle and intermittent daily use of tongue protectors for a two month period in a similar group of BMS patients. Parafunctional habits do not appear to have been assessed in the study population. Statistically significant improvements in pain intensity and quality of life were reported in the tongue protector group. As the authors recognise these results should be interpreted cautiously given the small number of patients, the short trial duration, the lack of blinding and absence of reported follow-up on completion of the study. Further information regarding the practicality of tongue protector use for BMS patients would be pertinent. The authors suggest that the tongue protector may be physically protecting the tongue mucosa from trauma but one could speculate on a more complex role in modifying pain transmission. Other chronic orofacial pain intervention studies have previously demonstrated the impact of a placebo effect on outcome4 and the authors recognise the possibility of a placebo effect contributing to the outcome of this study.
References
Zakrewska JM, Forssell H, Glenny AM . Interventions for the treatment of burning mouth syndrome. Cochrane Database Syst Rev. 2005; 25: CD002779
Zakrewska JM . Facial pain: an update. Curr Opinion Support Palliat Care. 2009; 3: 125–130
Lopez-Jornet P, Camacho-Alonso F, Leon-Espinosa S . Burning mouth syndrome, oral parafunctions, and psychological profile in a longitudinal case study. J Eur Acad Dermatol Venereol. 2009; 23: 363–365
Harrison SD, Glover L, Feinmann C, Pearce SA, Harris M . A comparison of antidepressant medication alone and in conjunction with cognitive behavior therapy for chronic idiopathic facial pain. In : Jensen TS, Turner JA, Wiesenfeld-Hallin Z eds Procedings of the 8th World Congress on Pain, Progress in Pain Research and Management. IASP Press, Seattle, WA, 1997; pp663–672.
Author information
Authors and Affiliations
Additional information
Address for Correspondence P López-Jornet, Department of Oral Medicine, University of Murcia, Murcia, Spain. Email: majornet@um.es
López-Jornet P, Camacho-Alonso F, Andujar-Mateos P. A prospective, randomized study on the efficacy of tongue protector in patients with burning mouth syndrome. Oral Dis. 2011; 17: 277–282
Rights and permissions
About this article
Cite this article
Buchanan, J. Tongue protectors for use in burning mouth syndrome?. Evid Based Dent 13, 59–60 (2012). https://doi.org/10.1038/sj.ebd.6400866
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ebd.6400866