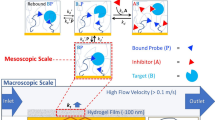
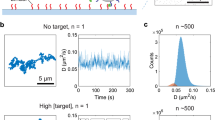
Abstract
Monitoring the kinetics of protein interactions on a high-density sensor array is vital to drug development and proteomic analysis. Label-free kinetic assays based on surface plasmon resonance are the current gold standard, but they have poor detection limits, suffer from non-specific binding, and are not amenable to high-throughput analyses. Here, we show that magnetically responsive nanosensors that have been scaled to over 100,000 sensors per cm2 can be used to measure the binding kinetics of various proteins with high spatial and temporal resolution. We present an analytical model that describes the binding of magnetically labelled antibodies to proteins that are immobilized on the sensor surface. This model is able to quantify the kinetics of antibody–antigen binding at sensitivities as low as 20 zeptomoles of solute.
This is a preview of subscription content, access via your institution
Access options




Similar content being viewed by others
References
Schena, M., Shalon, D., Davis, R. W. & Brown, P. O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270, 467–470 (1995).
MacBeath, G. & Schreiber, S. L. Printing proteins as microarrays for high-throughput function determination. Science 289, 1760–1763 (2000).
Zheng, G., Patolsky, F., Cui, Y., Wang, W. U. & Lieber, C. M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nature Biotechnol. 23, 1294–1301 (2005).
James, L. C. & Tawfik, D. S. Structure and kinetics of a transient antibody binding intermediate reveal a kinetic discrimination mechanism in antigen recognition. Proc. Natl Acad. Sci. USA 102, 12730–12735 (2005).
LaBaer, J. & Ramachandran, N. Protein microarrays as tools for functional proteomics. Curr. Opin. Chem. Biol. 9, 14–19 (2005).
Park, J. et al. A highly sensitive and selective diagnostic assay based on virus nanoparticles. Nature Nanotech. 4, 259–264 (2009).
Hudson, P. J. & Souriau, C. Engineered antibodies. Nature Med. 9, 129–134 (2003).
Schrama, D., Reisfeld, R. A. & Becker, J. C. Antibody targeted drugs as cancer therapeutics. Nature Rev. Drug Discov. 5, 147–159 (2006).
Sinensky, A. K. & Belcher, A. M. Label-free and high-resolution protein//DNA nanoarray analysis using kelvin probe force microscopy. Nature Nanotech. 2, 653–659 (2007).
Haab, B. B., Dunham, M. J. & Brown, P. O. Protein microarrays for highly parallel detection and quantitation of specific proteins and antibodies in complex solutions. Genome Biol. 2, research0004.1-research0004.13 (2001).
Wilson, W. D. Analyzing biomolecular interactions. Science 295, 2103–2105 (2002).
Bornhop, D. J. et al. Free-solution, label-free molecular interactions studied by back-scattering interferometry. Science 317, 1732–1736 (2007).
Stern, E. et al. Label-free biomarker detection from whole blood. Nature Nanotech. 5, 138–142 (2010).
Squires, T. M., Messinger, R. J. & Manalis, S. R. Making it stick: convection, reaction and diffusion in surface-based biosensors. Nature Biotechnol. 26, 417–426 (2008).
Ramachandran, N. et al. Self-assembling protein microarrays. Science 305, 86–90 (2004).
Patolsky, F. et al. Electrical detection of single viruses. Proc. Natl Acad. Sci. USA 101, 14017–14022 (2004).
Patolsky, F. & Lieber, C. M. Nanowire nanosensors. Materials Today 8, 20–28 (2005).
Berg, H. & Purcell, E. Physics of chemoreception. Biophys. J. 20, 193–219 (1977).
Berg, O. G. & von Hippel, P. H. Diffusion-controlled macromolecular interactions. Annu. Rev. Biophys. Biophys. Chem. 14, 131–160 (1985).
Stenberg, M. & Nygren, H. Kinetics of antigen–antibody reactions at solid–liquid interfaces. J. Immunol. Methods 113, 3–15 (1988).
Waite, B. A. & Stewart, J. D. An idealized dynamical model of simple diffusional interactions between macromolecules and between macromolecules and surfaces. Math. Biosci. 114, 173–213 (1993).
Sheehan, P. E. & Whitman, L. J. Detection limits for nanoscale biosensors. Nano Lett. 5, 803–807 (2005).
Swift, J. L. & Cramb, D. T. Nanoparticles as fluorescence labels: is size all that matters? Biophys. J. 95, 865–876 (2008).
Röcker, C., Pötzl, M., Zhang, F., Parak, W. J. & Nienhaus, G. U. A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nature Nanotech. 4, 577–580 (2009).
Osterfeld, S. J. et al. Multiplex protein assays based on real-time magnetic nanotag sensing. Proc. Natl Acad. Sci. USA 105, 20637–20640 (2008).
Gaster, R. S. et al. Matrix-insensitive protein assays push the limits of biosensors in medicine. Nature Med. 15, 1327–1332 (2009).
Gaster, R. S., Hall, D. A. & Wang, S. X. nanoLAB: an ultraportable, handheld diagnostic laboratory for global health. Lab Chip 11, 950–956 (2011).
Baibich, M. N. et al. Giant magnetoresistance of (001)Fe/(001)Cr magnetic superlattices. Phys. Rev. Lett. 61, 2472–2475 (1988).
Barnas, J., Fuss, A., Camley, R., Grünberg, P. & Zinn, W. Novel magnetoresistance effect in layered magnetic structures: theory and experiment. Phys. Rev. B 42, 8110–8120 (1990).
Prinz, G. A. Magnetoelectronics. Science 282, 1660–1663 (1998).
Wolf, S. A. et al. Spintronics: a spin-based electronics vision for the future. Science 294, 1488–1495 (2001).
Mulvaney, S., Myers, K., Sheehan, P. & Whitman, L. Attomolar protein detection in complex sample matrices with semi-homogeneous fluidic force discrimination assays. Biosens. Bioelectron. 24, 1109–1115 (2009).
Baselt, D. R. et al. A biosensor based on magnetoresistance technology. Biosens. Bioelectron. 13, 731–739 (1998).
Sandhu, A. Biosensing: new probes offer much faster results. Nature Nanotech. 2, 746–748 (2007).
Koets, M., van der Wijk, T., van Eemeren, J., van Amerongen, A. & Prins, M. Rapid DNA multi-analyte immunoassay on a magneto-resistance biosensor. Biosens. Bioelectron. 24, 1893–1898 (2009).
Xu, L. et al. Giant magnetoresistive biochip for DNA detection and HPV genotyping. Biosens. Bioelectron. 24, 99–103 (2008).
Koh, A. L. & Sinclair, R. TEM studies of iron oxide nanoparticles for cell labeling and magnetic separation. Technical Proceedings of the 2007 NSTI Nanotechnology Conference and Trade Show, 101–104 (2007).
De Palma, R. et al. Magnetic particles as labels in bioassays: interactions between a biotinylated gold substrate and streptavidin magnetic particles. J. Phys. Chem. C 111, 12227–12235 (2007).
Malmqvist, M. Biospecific interaction analysis using biosensor technology. Nature 361, 186–187 (1993).
Katsamba, P. S. et al. Kinetic analysis of a high-affinity antibody/antigen interaction performed by multiple Biacore users. Anal. Biochem. 352, 208–221 (2006).
Lausted, C., Hu, Z. & Hood, L. Quantitative serum proteomics from surface plasmon resonance imaging. Mol. Cell Proteomics 7, 2464–2474 (2008).
Rich, R. L. & Myszka, D. G. Higher-throughput, label-free, real-time molecular interaction analysis. Anal. Biochem. 361, 1–6 (2007).
Campbell, C. T. & Kim, G. SPR microscopy and its applications to high-throughput analyses of biomolecular binding events and their kinetics. Biomaterials 28, 2380–2392 (2007).
Wang, S. & Guanxiong Li. Advances in giant magnetoresistance biosensors with magnetic nanoparticle tags: review and outlook. IEEE Trans. Magnetics 44, 1687–1702 (2008).
Myszka, D. G., He, X., Dembo, M., Morton, T. A. & Goldstein, B. Extending the range of rate constants available from BIACORE: interpreting mass transport-influenced binding data. Biophys. J 75, 583–594 (1998).
Reichert, J. M. & Valge-Archer, V. E. Development trends for monoclonal antibody cancer therapeutics. Nature Rev. Drug Discov. 6, 349–356 (2007).
Willett, C. G. et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nature Med. 10, 145–147 (2004).
Anderson, N. L. The clinical plasma proteome: a survey of clinical assays for proteins in plasma and serum. Clin. Chem. 56, 177–185 (2010).
Cai, W. et al. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 6, 669–676 (2006).
Gaster, R. S., Hall, D. A. & Wang, S. X. Autoassembly protein arrays for analyzing antibody cross-reactivity. Nano Lett. doi:10.1021/nl1026056 (2010).
Srisa-Art, M., Dyson, E. C., deMello, A. J. & Edel, J. B. Monitoring of real-time streptavidin–biotin binding kinetics using droplet microfluidics. Anal. Chem. 80, 7063–7067 (2008).
Barbosa, M. D., Chamberlain, A. K. & Desjarlais, J. R. Optimized proteins that target Ep-CAM. US patent 7,557,190 (2009), available via http://go.nature.com/lbOXuD
Hefta, L. J. F., Neumaier, M. & Shively, J. E. Kinetic and affinity constants of epitope specific anti-carcinoembryonic antigen (CEA) monoclonal antibodies for CEA and engineered CEA domain constructs. Immunotechnology 4, 49–57 (1998).
Chen, Y. et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured fab in complex with antigen. J. Mol. Biol. 293, 865–881 (1999).
Acknowledgements
This work was supported, in part, by the United States National Cancer Institute (grants 1U54CA119367, 1U54CA143907, 1U54CA151459 and N44CM–2009-00011), the United States National Science Foundation (grant ECCS-0801385-000), the United States Defense Advanced Research Projects Agency/Navy (grant N00014–02-1–0807), a Gates Foundation Grand Challenge Exploration Award and The National Semiconductor Corporation. R.S.G. acknowledges financial support from the Stanford Medical School Medical Scientist Training Program and a National Science Foundation graduate research fellowship. The authors thank M. Hammer and A. Bhattacharjee for editing the manuscript.
Author information
Authors and Affiliations
Contributions
R.S.G. and S.X.W designed the research. R.S.G. performed the research. R.S.G., R.J.W. and S.X.W developed the model. R.S.G., L.X., S.H., R.J.W., D.A.H., S.J.O., H.Y. and S.X.W. contributed analytical tools. R.S.G., R.J.W. and S.X.W analysed the data. S.J.O., L.X., S.H. and S.X.W. designed the magnetic sensor arrays. R.S.G. and H.Y. developed the biochemistry. R.S.G. and S.X.W. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
Stanford University has licensed part of the magnetic bioassay chip technology contained in this publication to MagArray Inc., an early stage startup company in Silicon Valley, USA. S.X.W, H.Y., and S.J.O. hold financial interests in MagArray in the form of stock options.
Supplementary information
Supplementary information
Supplementary information (PDF 1486 kb)
Rights and permissions
About this article
Cite this article
Gaster, R., Xu, L., Han, SJ. et al. Quantification of protein interactions and solution transport using high-density GMR sensor arrays. Nature Nanotech 6, 314–320 (2011). https://doi.org/10.1038/nnano.2011.45
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2011.45
This article is cited by
-
Highly stable integration of graphene Hall sensors on a microfluidic platform for magnetic sensing in whole blood
Microsystems & Nanoengineering (2023)
-
A tandem giant magnetoresistance assay for one-shot quantification of clinically relevant concentrations of N-terminal pro-B-type natriuretic peptide in human blood
Analytical and Bioanalytical Chemistry (2021)
-
Open-shell organic semiconductors: an emerging class of materials with novel properties
Polymer Journal (2018)
-
Discrete microfluidics for the isolation of circulating tumor cell subpopulations targeting fibroblast activation protein alpha and epithelial cell adhesion molecule
npj Precision Oncology (2017)
-
Multiplex giant magnetoresistive biosensor microarrays identify interferon-associated autoantibodies in systemic lupus erythematosus
Scientific Reports (2016)