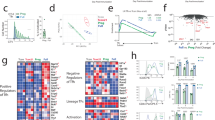
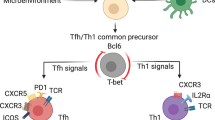
Abstract
Understanding the developmental mechanisms of follicular helper T cells (TFH cells) in humans is relevant to the clinic. However, the factors that drive the differentiation of human CD4+ helper T cells into TFH cells remain largely undefined. Here we found that transforming growth factor-β (TGF-β) provided critical additional signals for the transcription factors STAT3 and STAT4 to promote initial TFH differentiation in humans. This mechanism did not appear to be shared by mouse helper T cells. Developing human TFH cells that expressed the transcriptional repressor Bcl-6 also expressed RORγt, a transcription factor typically expressed by the TH17 subset of helper T cells. Our study documents a mechanism by which TFH cells and TH17 cells emerge together in inflammatory environments in humans, as is often observed in many human autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011).
Victora, G.D. & Nussenzweig, M.C. Germinal centers. Annu. Rev. Immunol. 30, 429–457 (2012).
Bossaller, L. et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J. Immunol. 177, 4927–4932 (2006).
Choi, Y.S. et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34, 932–946 (2011).
Xu, H. et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 496, 523–527 (2013).
Vogelzang, A. et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29, 127–137 (2008).
Bentebibel, S.E., Schmitt, N., Banchereau, J. & Ueno, H. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc. Natl. Acad. Sci. USA 108, E488–E497 (2011).
Craft, J.E. Follicular helper T cells in immunity and systemic autoimmunity. Nat. Rev. Rheumatol. 8, 337–347 (2012).
Vinuesa, C.G. & Cyster, J.G. How T cells earn the follicular rite of passage. Immunity 35, 671–680 (2011).
Baumjohann, D., Okada, T. & Ansel, K.M. Cutting edge: distinct waves of BCL6 expression during T follicular helper cell development. J. Immunol. 187, 2089–2092 (2011).
Fazilleau, N., Mark, L., McHeyzer-Williams, L.J. & McHeyzer-Williams, M.G. Follicular helper T cells: lineage and location. Immunity 30, 324–335 (2009).
Nakayamada, S. et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 35, 919–931 (2011).
Johnston, R.J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
Nurieva, R.I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009).
Yu, D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009).
Sweet, R.A., Lee, S.K. & Vinuesa, C.G. Developing connections amongst key cytokines and dysregulated germinal centers in autoimmunity. Curr. Opin. Immunol. 24, 658–664 (2012).
Schmitt, N. et al. IL-12 receptor β1 deficiency alters in vivo T follicular helper cell response in humans. Blood 121, 3375–3385 (2013).
Schmitt, N. et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity 31, 158–169 (2009).
Ma, C.S. et al. Early commitment of naive human CD4+ T cells to the T follicular helper (TFH) cell lineage is induced by IL-12. Immunol. Cell Biol. 87, 590–600 (2009).
Schraml, B.U. et al. The AP-1 transcription factor Batf controls TH17 differentiation. Nature 460, 405–409 (2009).
Ise, W. et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat. Immunol. 12, 536–543 (2011).
Murphy, T.L., Tussiwand, R. & Murphy, K.M. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat. Rev. Immunol. 13, 499–509 (2013).
Li, M.O. & Flavell, R.A. TGF-β: a master of all T cell trades. Cell 134, 392–404 (2008).
Manel, N., Unutmaz, D. & Littman, D.R. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat. Immunol. 9, 641–649 (2008).
Trinchieri, G., Pflanz, S. & Kastelein, R.A. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19, 641–644 (2003).
Ma, C.S. et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood 119, 3997–4008 (2012).
Chtanova, T. et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 173, 68–78 (2004).
Nurieva, R.I. et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008).
Takahashi, H. et al. TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat. Immunol. 13, 587–595 (2012).
Suto, A. et al. Development and characterization of IL-21-producing CD4+ T cells. J. Exp. Med. 205, 1369–1379 (2008).
Lu, K.T. et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity 35, 622–632 (2011).
Eto, D. et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE 6, e17739 (2011).
Kallies, A. et al. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat. Immunol. 7, 466–474 (2006).
Ray, J.P. et al. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 Cells. Immunity 40, 367–377 (2014).
Banchereau, J. & Pascual, V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25, 383–392 (2006).
Le Bon, A. et al. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14, 461–470 (2001).
Cucak, H., Yrlid, U., Reizis, B., Kalinke, U. & Johansson-Lindbom, B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity 31, 491–501 (2009).
Morita, R. et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121 (2011).
Boswell, K.L. et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog. 10, e1003853 (2014).
Li, X.Y. et al. Role of the frequency of blood CD4+CXCR5+CCR6+ T cells in autoimmunity in patients with Sjogren's syndrome. Biochem. Biophys. Res. Commun. 422, 238–244 (2012).
Romme Christensen, J. et al. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS ONE 8, e57820 (2013).
Le Coz, C. et al. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS ONE 8, e75319 (2013).
Singh, N. & Cohen, P.L. The T cell in Sjogren's syndrome: force majeure, not spectateur. J. Autoimmun. 39, 229–233 (2012).
Nylander, A. & Hafler, D.A. Multiple sclerosis. J. Clin. Invest. 122, 1180–1188 (2012).
Buckley, C.D. et al. Persistent induction of the chemokine receptor CXCR4 by TGF-β1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J. Immunol. 165, 3423–3429 (2000).
Ciofani, M. et al. A validated regulatory network for Th17 cell specification. Cell 151, 289–303 (2012).
Acknowledgements
We thank E. Trahan, S. Coquery, N. Loof and K. Kayembe for cell sorting; K. Palucka, V. Pascual and Y.-J. Liu for discussions; S. Clayton for confocal imaging; C. Quinn for development of the heat map–generation software; Y. Kanno for advice about chromatin immunoprecipitation; Y.C. Lin for discussions about chromatin-immunoprecipitation data analysis; and M. Kathania for technical help with mouse experiments. Supported by the US National Institutes of Health (U19-AI057234, U19-AI082715 and U19-AI089987), the Alliance for Lupus Research, the Baylor Health Care System (H.U.) and the American Cancer Society (122713-RSG-12-260-01-LIB to K.V.).
Author information
Authors and Affiliations
Contributions
N.S. conceived of and did experiments and analyzed the data; Y.L. and L.B. stained tonsillar tissue; S.-E.B. contributed to the preparation of tonsillar helper T cells; I.M. did NanoString assays; K.V. contributed to experiments with mouse cells; H.U. oversaw and conceived of the entire project and analyzed the data; and N.S., J.B. and H.U. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 TGF-β plus IL-12 and TGF-β plus IL-23 induce the expression of TFH cell–related molecules by human naive helper T cells.
(a) Cell viability and cell recovery of human adult blood naive TH cells following 4 days of stimulation with CD3-CD28 mAbs in the presence of the indicated cytokines during the last 3 days. Recovered viable cell numbers are shown in a cell recovery index determined by this formula: recovered cell numbers in the culture with the indicated cytokines / recovered cell numbers in the culture with no cytokine. Each dot represents a result from 10 sets of 4 d-culture experiments. Black bars show the mean value and the blue boxes show the range of ± 1 s.d. (b) Expression of IL-2, IL-21, and ICOS by activated (CFSE-) human naive CFSE-labeled TH cells cultured with no cytokine, IL-12, and IL-12 + IL-4 as in (a). A representative out of 2 experiments. (c) Expression of IL-2, and IL-21 by activated (FSChiSSChi) human naive TH cells cultured with no cytokine, IL-12, IL-12 + TGF-β, and IL-12 + TGF-β + IL-4 as in (a). A representative out of 2 experiments. (d) Expression of CXCR5 and ICOS on activated human naive TH cells stimulated with the indicated cytokines as in (a). Results are from one representative of 13 sets of 4 d-culture experiments.
Supplementary Figure 2 TGF-β plus IL-12 and TGF-β plus IL-23 promote the induction of the TFH cell transcriptional signature.
(a) Isolation of four subsets from ex vivo human tonsillar TH cells according to the expression of CXCR5 and ICOS (gated to CD4+ T cells). (b) Expression of transcription factors by the four tonsillar TH subsets. Data were obtained from TH subsets isolated from 2-3 tonsil samples. (c) Ranking of the differentiation conditions inducing the high ratio of BCL6 to PRDM1 by human adult blood naive TH cells cultured with various cytokines (CK) as in Fig. 2a. The data were normalized to the values in the culture with no cytokines in each experiment. Each dot represents a result from 11 sets of 4 days culture experiments. Black bars show the mean value and blue boxes indicate ± 1 s.d. (d) Ranking of the differentiation conditions inducing the expression of BCL6, BATF, JUN, JUNB, the high ratio of BCL6 to PRDM1, and suppressing the expression of PRDM1 by human cord blood naive TH cells cultured with various cytokines (CK) as in Fig. 2a. Each dot represents a result from 4 sets of 4 days culture experiments.
Supplementary Figure 3 TGF-β plus IL-12 and TGF-β plus IL-23 promote induction of the TFH cell transcriptional signature.
(a) Ranking of the differentiation conditions inducing the expression of MAF and BATF by human adult blood naive TH cells cultured with various cytokines (CK) as in Fig. 1a. Each dot represents a result from 11 sets of 4 days culture experiments. (b) Correlation between BCL6 and MAF, BATF, and PRDM1 expressed by human cord blood naive TH cells cultured as in (a). Pearson R values are indicated. P values were all <0.0001. (c) Two predicted BATF binding sites in the BCL6 gene are shown in the UCSC Genome Browser view. Primer sets for the binding sites #1 and #2 are indicated in the table. (d) Expression of CXCR5, ICOS, IL-21, and Bcl-6 by activated (FSChiSSChi) adult blood naive TH cells stimulated for 4 days with allogeneic CD40L-stimulated DCs in the presence of the indicated amounts of TGF-β. A representative result from 3 independent experiments. Results from 3 independent experiments are shown in panel (e).
Supplementary Figure 4 Expression of BCL6 in cultured cord blood naive helper T cells positively correlates with the expression of AHR, RORA, and RORC.
Correlation between BCL6 and the indicated transcription factors expressed by human cord blood naive TH cells cultured for 3 days with different cytokines as in Fig. 2a. Pearson R values and p values are indicated.
Supplementary Figure 5 RORγt is expressed by CXCR5+Bcl-6+ TH cells generated with TGF-β plus IL-12 or with TGF-β plus IL-23.
(a) The expression of Bcl-6, RORγt, and T-bet by activated (FSChiSSChi) human adult blood naive TH cells cultured with the indicated cytokines for 3 days as in Fig. 1a. A representative of 4 independent experiments. (b) The expression of Bcl-6, RORγt, and T-bet by CXCR5+ TH cells generated by culturing naive TH cells with the indicated cytokines for 3 days. A representative of 4 independent experiments. (c) The expression of Bcl-6 and RORγt by CXCR5+ human adult blood naive TH cells cultured with the indicated cytokines for 3 days as in Fig. 1a. A representative of 3 independent experiments. (d) The expression of Bcl-6, RORγt, and T-bet by activated (FSChiSSChi) human adult blood naive TH cells transfected with either scrambled or RORγt siRNA followed by 2 days of culture with no cytokines or IL-23 + IL-1β + IL-6 + TGF-β. A representative of 3 independent experiments. (e) The expression of IL-17A and IL-21 by activated (FSChiSSChi) cord blood naive TH cells cultured for 3 days with no cytokines or IL-23 + IL-1β + IL-6 + TGF-β. A representative of 3 independent experiments. The frequency of IL-21+ cells among IL-17A+ cells was 38 ± 2% (Mean ± s.d., n=3).
Supplementary Figure 6 A small fraction of GC TFH cells express T-bet but not GATA-3.
(a) Expression of transcription factors by the four tonsillar TH subsets (Mean ± s.e.m, n=3). (b) Human tonsil sections were stained for IgD (indicated by green), CD4 (indicated by blue), and Bcl-6 (indicated by red), and analyzed by confocal microscopy. Scale bar, 100 μm. Representative Bcl-6+ TH cells in GCs are shown in panel (c). Scale bar, 10 μm. (d) The expression of Bcl-6, T-bet, and Gata3 by ex vivo tonsillar CXCR5-ICOS- naive TH cells and CXCR5hiICOShi GC TFH cells. A representative of 4 independent experiments.
Supplementary Figure 7 TGF-β inhibits the expression of IL-21 and ICOS by mouse helper T cells.
(a) Cell viability and cell recovery of mouse naive TH cells following 4 days of stimulation with CD3-CD28 mAbs in the presence of the indicated cytokines during the last 3 days. Recovered viable cell numbers are shown in a cell recovery index determined by this formula: recovered cell numbers in the culture with the indicated cytokines / recovered cell numbers in the culture with no cytokine. Each dot represents a result from 5 sets of 4 d-culture experiments. Black bars show the mean value and the blue boxes show the range of ± 1 s.d. (b) The expression of IL-17, IL-21, CXCR5, and ICOS by activated (FSChiSSChi) mouse naive TH cells cultured for 3 days with the indicated amounts of TGF-β plus IL-12 + IL-6 + IL-21 or plus IL-23 + IL-6 + IL-21 as in (a). A representative of 4 independent experiments. Results from 5 sets of experiments are shown in panel (c). (d) CXCR5 expression by B cells in Spleen and lymph nodes cells analyzed with the anti-mouse CXCR5 mAb used for the phenotyping of mouse TH cells.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 6404 kb)
Rights and permissions
About this article
Cite this article
Schmitt, N., Liu, Y., Bentebibel, SE. et al. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol 15, 856–865 (2014). https://doi.org/10.1038/ni.2947
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2947
This article is cited by
-
Role and Interrelationship Between Myeloid-Derived Suppressor Cells and CD4+ T Cells in Different Types of Infections: A Review
Proceedings of the Zoological Society (2024)
-
Iguratimod suppresses Tfh cell differentiation in primary Sjögren’s syndrome patients through inhibiting Akt/mTOR/STAT3 signaling
Arthritis Research & Therapy (2023)
-
Lipid nanoparticles (LNP) induce activation and maturation of antigen presenting cells in young and aged individuals
Communications Biology (2023)
-
TGF-β in correlation with tumor progression, immunosuppression and targeted therapy in colorectal cancer
Medical Oncology (2023)
-
TSLP promoting B cell proliferation and polarizing follicular helper T cell as a therapeutic target in IgG4-related disease
Journal of Translational Medicine (2022)