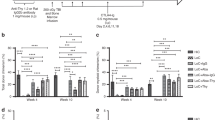
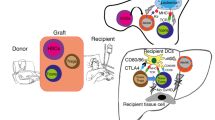
Summary:
To investigate whether we could create a radiation-free conditioning regimen to induce permanent mixed and multilineage chimerism and donor-specific tolerance, we treated recipient mice with anti-T-cell antibodies, varying and fractionated doses of Treosulfan and fully MHC disparate bone marrow cells. Treosulfan is mainly used in the treatment of ovarian cancer. It is a structural analog of busulfan, but it does not induce severe hepatotoxicity or veno-occlusive disease at or above the maximum tolerated dose, lacks significant nonhematological toxicity and has limited organ toxicity. We report here the successful induction of permanent mixed multilineage chimerism and donor-specific tolerance as was proven by skin transplantation and IFN-γ ELISPOT. In conclusion, because of its lower nonhematological toxicity, compared with other myeloablative regimens (eg irradiation or busulfan admin- istration), Treosulfan could be a better candidate for conditioning to induce donor-specific allograft tolerance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Glover MT, Deeks JJ, Raftery MJ et al. Immunosuppression and risk of non-melanoma skin cancer in renal transplant recipients. Lancet 1997; 349: 398.
Penn I . Occurrence of cancers in immunosuppressed organ transplant recipients. In: Cecka GM, Terosaki PI (eds) Clinical Transplants. Tissue Typing Laboratory, Los Angeles, Califorrnia, USA, 1998; 147–158.
Falkenhain ME, Cosio FG, Sedmak DD . Progressive histologic injury in kidneys from heart and liver transplant recipients receiving cyclosporine. Transplantation 1996; 62: 364–370.
de Vries-van der Zwan, Besseling AC, van der Pol MA et al. Specific tolerance induction and organ transplantation. Leukemia Lymphoma 1998; 31: 131–142.
de Vries-van der Zwan, Besseling AC, de Waal LP et al. Specific tolerance induction and transplantation: a single-day protocol. Blood 1997; 89: 2596–2601.
Sharabi Y, Sachs DH . Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med 1989; 169: 493–502.
Wekerle T, Sykes M . Mixed chimerism as an approach for the induction of transplantation tolerance. Transplantation 1999; 68: 459–467.
Jones TR, Ha J, Williams MA et al. The Role of the IL-2 pathway in costimulation blockade-resistant rejection of allografts. J Immunol 2002; 168: 1123–1130.
Maier S, Tertilt C, Chambron N et al. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28−/− mice. Nat Med 2001; 7: 557–562.
de Vries-van der Zwan, Besseling AC, Kievits F et al. Anti-CD3 treatment facilitates engraftment of full H-2-disparate donor bone marrow cells and subsequent skin allograft tolerance. Transplantation 1994; 58: 610–617.
Hartley JA, O'Hare CC, Baumgart J . DNA alkylation and interstrand cross-linking by treosulfan. Br J Cancer 1999; 79: 264–266.
Scheulen ME, Hilger RA, Oberhoff C et al. Clinical phase I dose escalation and pharmacokinetic study of high-dose chemotherapy with treosulfan and autologous peripheral blood stem cell transplantation in patients with advanced malignancies. Clin Cancer Res 2000; 6: 4209–4216.
Rigos D, Wechsel HW, Bichler KH . Treosulfan in the treatment of metastatic renal cell carcinoma. Anticancer Res 1999; 19: 1549–1552.
Kopf-Maier P, Sass G . Antitumor activity of treosulfan in human lung carcinomas. Cancer Chemother Pharmacol 1996; 37: 211–221.
Gropp M, Meier W, Hepp H . Treosulfan as an effective second-line therapy in ovarian cancer. Gynecol Oncol 1998; 71: 94–98.
Neuber K, Tom DA, Blodorn-Schlicht N et al. Treosulfan is an effective alkylating cytostatic for malignant melanoma in vitro and in vivo. Melanoma Res 1999; 9: 125–132.
Kopf-Maier P, Sass G . Antitumor activity of treosulfan against human breast carcinomas. Cancer Chemother Pharmacol 1992; 31: 103–110.
Fichtner I, Becker M, Baumgart J . Antileukaemic activity of treosulfan in xenografted human acute lymphoblastic leukemias (ALL) Eur J Cancer 2003; 39: 801–807.
Meden H, Wittkop Y, Kuhn W . Maintenance chemotherapy with oral treosulfan following first-line treatment in patients with advanced ovarian cancer: feasibility and toxicity. Anti-cancer Res 1997; 17: 2221–2223.
Leo O, Foo M, Sachs DH et al. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci USA 1987; 84: 1374–1378.
Ploemacher RE, van der Sluijs JP, Voerman JS et al. An in vitro limiting-dilution assay of long-term repopulating hematopoietic stem cells in the mouse. Blood 1989; 74: 2755–2763.
Ferran C, Sheehan K, Dy M et al. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur J Immunol 1990; 20: 509–515.
de Vries-van der Zwan, van der Pol MA, Besseling AC et al. Haematopoietic stem cells can induce specific skin graft acceptance across full MHC barriers. Bone Marrow Transplant 1998; 22: 91–98.
Okada S, Nakauchi H, Nagayoshi K et al. Enrichment and characterization of murine hematopoietic stem cells that express c-kit molecule. Blood 1991; 78: 1706–1712.
Kaufman CL, Colson YL, Wren SM et al. Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood 1994; 84: 2436–2446.
Gandy KL, Domen J, Aguila H et al. CD8+TCR+ and CD8+TCR− cells in whole bone marrow facilitate the engraftment of hematopoietic stem cells across allogeneic barriers. Immunity 1999; 11: 579–590.
Uchida N, Weissman IL . Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin− Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J Exp Med 1992; 175: 175–184.
Gandy KL, Weissman IL . Tolerance of allogeneic heart grafts in mice simultaneously reconstituted with purified allogeneic hematopoietic stem cells. Transplantation 1998; 65: 295–304.
Hashimoto F, Sugiura K, Inoue K et al. Major histocompatibility complex restriction between hematopoietic stem cells and stromal cells in vivo. Blood 1997; 89: 49–54.
Zeiger E, Pagano DA . Mutagenicity of the human carcinogen treosulphan in Salmonella. Environ Mol Mutagen 1989; 13: 343–346.
Westerhof GR, Blokland I, Down JD et al. In vivo and in vitro sensitivity of murine bone marrow hematopoietic progenitors for treosulfan. Blood 1998; 92: 197b.
Westerhof GR, Ploemacher RE, Boudewijn A et al. Comparison of different busulfan analogues for depletion of hematopoietic stem cells and promotion of donor-type chimerism in murine bone marrow transplant recipients. Cancer Res 2000; 60: 5470–5478.
Ploemacher RE, Westerhof GR, Blokland I et al. Treosulfan as an alternative conditioning agent in bone marrow transplantation. Bone Marrow Transplant 2000; 25: abstract no P421.
Adams AB, Durham MM, Kean L et al. Costimulation blockade, busulfan, and bone marrow promote titratable macrochimerism, induce transplantation tolerance, and correct genetic hemoglobinopathies with minimal myelosuppression. J Immunol 2001; 167: 1103–1111.
Leong LY, Qin S, Cobbold SP et al. Classical transplantation tolerance in the adult: the interaction between myeloablation and immunosuppression. Eur J Immunol 1992; 22: 2825–2830.
Tomita Y, Yoshikawa M, Zhang QW et al. Induction of permanent mixed chimerism and skin allograft tolerance across fully MHC-mismatched barriers by the additional myelosuppressive treatments in mice primed with allogeneic spleen cells followed by cyclophosphamide. J Immunol 2000; 165: 34–41.
de Vries-van der Zwan, van der Pol MA, de Waal LP et al. An alternative conditioning regimen for induction of specific skin graft tolerance across full major histocompatibility complex barriers. Transplant Immunol 1998; 6: 147–151.
Beelen DW . Evaluation of safety efficacy and pharmacokinetics of dose-escalated treosulfan (TREO)/cyclophosohamide (CY) conditioning prior to allogeneic transplantation of high-risk leukemia patients. Blood 2002; 100: 415a, absract no. 1608.
Casper J, knauf W, Doelken G et al. Treosulfan and fludarabine as conditioning for allogeneic blood stem cell transplantation-final analysis of a phase I/II study. Onkologie 2002; 54: 93, absract 320.
Acknowledgements
We thank Sacco Luypen and Mathijs van Eck for assistance in the animal experiments and animal care, and Joachim Baumgart (Medac) for a critical reading of the manuscript. This study was financially supported by the Dutch Kidney Foundation (Grant No. C97.1669).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Pel, M., van Breugel, D., Vos, W. et al. Towards a myeloablative regimen with clinical potential: I. Treosulfan conditioning and bone marrow transplantation allow induction of donor-specific tolerance for skin grafts across full MHC barriers. Bone Marrow Transplant 32, 15–22 (2003). https://doi.org/10.1038/sj.bmt.1704094
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704094
Keywords
This article is cited by
-
Treosulfan-based conditioning regimens for allogeneic HSCT in children with acute lymphoblastic leukaemia
Annals of Hematology (2015)
-
Treosulfan-based conditioning before hematopoietic SCT: more than a BU look-alike
Bone Marrow Transplantation (2012)
-
Substitution of cyclophosphamide and busulfan by fludarabine, treosulfan and melphalan in a preparative regimen for children and adolescents with Shwachman–Diamond syndrome
Bone Marrow Transplantation (2007)
-
Dose-escalated treosulphan in combination with cyclophosphamide as a new preparative regimen for allogeneic haematopoietic stem cell transplantation in patients with an increased risk for regimen-related complications
Bone Marrow Transplantation (2005)
-
Toward a myeloablative regimen with clinical potential: II. Treosulfan induces specific skin graft tolerance across haploidentical MHC barriers
Bone Marrow Transplantation (2004)