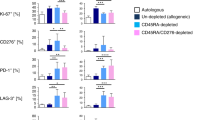
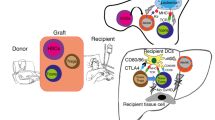
Abstract
The use of haploidentical HSCT is gaining momentum using either megadose T-cell depleted (TCD) myeloablative HSCT, or T cell replete nonmyeloablative HSCT in conjunction with cyclophosphamide post-transplant (PTCY). However, the risks of myeloablative protocols in the former or prolonged immunosuppression in the later, adversely impacting GVL and anti-pathogen immunity are challenging. Recently, we demonstrated in a stringent BM allografting murine model, that combining megadose TCD HSCT with PTCY, enabled durable chimerism induction without GVHD in the absence of any immune suppression after CY. The outcome of this approach in one a patient with multiple myeloma and one with Hodgkin’s disease indicate it might offer an attractive safe protocol for haploidentical HSCT. In addition, preclinical experiments show that donor type anti-third party central memory veto T cells that are administrated with a megadose TCD HSCT can potentially offer an editional tool for safer conditioning protocols. Moreover, proof of concept murine studies demonstrate that genetically manipulated veto cells armed with specific receptors against cancer cells, can survive for months in allogeneic recipients, laying the rational for the use of “off-the shelf ” Veto-CAR T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13:10.
Or‐Geva N, Reisner Y. The evolution of T‐cell depletion in haploidentical stem‐cell transplantation. Br J Haematol. 2016;172:667–84.
Spitzer TR, Mcafee SL, Dey BR, Colby C, Hope J, Grossberg H, et al. Nonmyeloablative haploidentical stem-cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation. 2003;75:1748–51.
Aversa F, Bachar-Lustig E, Or-Geva N, Prezioso L, Bonomini S, Manfra I, et al. Immune tolerance induction by nonmyeloablative haploidentical HSCT combining T-cell depletion and posttransplant cyclophosphamide. Blood Adv. 2017;1:2166–75.
Sachs DH, Kawai T, Sykes M. Induction of tolerance through mixed chimerism. Cold Spring Harb Perspect Med. 2014;4:a015529.
Sykes M. Immune tolerance: mechanisms and application in clinical transplantation. J Intern Med. 2007;262:288–310.
Rachamim N, Gan J, Segall H, Krauthgamer R, Marcus H, Berrebi A, et al. Tolerance induction by “megadose” hematopoietic transplants: donor-type human CD34 stem cells induce potent specific reduction of host anti-donor cytotoxic T lymphocyte precursors in mixed lymphocyte culture1. Transplantation. 1998;65:1386–93.
Miller RG. An immunological suppressor cell inactivating cytotoxic T-lymphocyte precursor cells recognizing it. Nature. 1980;287:544.
Zangi L, Klionsky YZ, Yarimi L, Bachar-Lustig E, Eidelstein Y, Shezen E, et al. Deletion of cognate CD8 T cells by immature dendritic cells: a novel role for perforin, granzyme A, TREM-1, and TLR7. Blood. 2012;120:1647–57.
Chrobak P, Gress RE. Veto activity of activated bone marrow does not require perforin and Fas ligand. Cell Immunol. 2001;208:80–7.
Reich-Zeliger S, Gan J, Bachar-Lustig E, Reisner Y. Tolerance induction by veto CTLs in the TCR transgenic 2C mouse model. II. Deletion of effector cells by Fas-Fas ligand apoptosis. J Immunol. 2004;173:6660–6.
Gur H, Krauthgamer R, Bachar-Lustig E, Katchman H, Arbel-Goren R, Berrebi A, et al. Immune regulatory activity of CD34 + progenitor cells: evidence for a deletion-based mechanism mediated by TNF-alpha. Blood. 2005;105:2585–93.
Reich-Zeliger S, Zhao Y, Krauthgamer R, Bachar-Lustig E, Reisner Y. Anti-third party CD8 CTLs as potent veto cells: coexpression of CD8 and FasL is a prerequisite. Immunity. 2000;13:507–15.
Milstein O, Hagin D, Lask A, Reich-Zeliger S, Shezen E, Ophir E, et al. CTLs respond with activation and granule secretion when serving as targets for T-cell recognition. Blood. 2011;117:1042–52.
Zlotnikov-Klionsky Y, Nathansohn-Levi B, Shezen E, Rosen C, Kagan S, Bar-On L, et al. Perforin-positive dendritic cells exhibit an immuno-regulatory role in metabolic syndrome and autoimmunity. Immunity. 2015;43:776–87.
Reich-Zeliger S, Bachar-Lustig E, Bar-Ilan A, Reisner Y. Tolerance induction in presensitized bone marrow recipients by veto CTLs: effective deletion of host anti-donor memory effector cells. J Immunol. 2007;179:6389–94.
Bachar-Lustig E, Reich-Zeliger S, Reisner Y. Anti-third-party veto CTLs overcome rejection of hematopoietic allografts: synergism with rapamycin and BM cell dose. Blood. 2003;102:1943–50.
Anderson KM, Zimring JC. Rapamycin prolongs susceptibility of responding T cells to tolerance induction by CD8 veto cells. Transplantation. 2006;81:88–94.
Ophir E, Eidelstein Y, Afik R, Bachar-Lustig E, Reisner Y. Induction of tolerance to bone marrow allografts by donor-derived host nonreactive ex vivo-induced central memory CD8 T cells. Blood. 2010;115:2095–104.
Or-Geva N, Gidron-Budovsky R, Sidlik-Muskatel R, Singh AK, Reisner Y. Next-generation CD8 memory veto T cells directed against memory antigens. Leukemia. 2019 Jun 12. [Epub ahead of print]
Ophir E, Or-Geva N, Gurevich I, Tal O, Eidelstein Y, Shezen E, et al. Murine anti-third-party central-memory CD8(+) T cells promote hematopoietic chimerism under mild conditioning: lymph-node sequestration and deletion of anti-donor T cells. Blood. 2013;121:1220–8.
Or-Geva N, Gidron-Budovsky R, Radomir L, Edelstein Y, Singh AK, Sidlik-Muskatel R, et al. Towards ‘off-the-shelf’genetically modified T cells: prolonging functional engraftment in mice by CD8 veto T cells. Leukemia. 2018;32:1038.
Funding
This article was supported in part by Cancer Prevention & Research Institute of Texas and by Cell Source LTD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
YR received grant funding, consulting fees and owns equity in Cell Source Ltd. RS-M declare that she has no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sidlik-Muskatel, R., Reisner, Y. Toward safer haploidnetical hematopoietic stem cell transplantation. Bone Marrow Transplant 54 (Suppl 2), 733–737 (2019). https://doi.org/10.1038/s41409-019-0595-0
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0595-0
This article is cited by
-
Allogeneic hematopoietic cell transplantation for patients with AML aged 70 years or older in first remission. A study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT)
Bone Marrow Transplantation (2023)
-
Which is better, HLA-matched sibling or haploidentical transplantation?
Cellular & Molecular Immunology (2021)