Abstract
Background:
Sunitinib is one of the first-line standard treatments for metastatic clear cell renal cell carcinoma (ccRCC) with a median time to progression shorter than 1 year. The objective is to discover predictive markers of response to adapt the treatment at diagnosis.
Methods:
Prospective phase 2 multi-centre trials were conducted in ccRCC patients initiating sunitinib (54 patients) or bevacizumab (45 patients) in the first-line metastatic setting (SUVEGIL and TORAVA trials). The plasmatic level of CXCL7 at baseline was correlated with progression-free survival (PFS).
Results:
The cut-off value of CXCL7 for PFS was 250 ng ml−1. Patients with CXCL7 plasmatic levels above the cut-off at baseline (250 ng ml−1) had a significantly longer PFS (hazard ratio 0.323 (95% confidence interval 0.147–0.707), P=0.001). These results were confirmed in a retrospective validation cohort. The levels of CXCL7 did not influence PFS of the bevacizumab-treated patients.
Conclusions:
CXCL7 may be considered as a predictive marker of sunitinib efficacy for ccRCC patients.
Similar content being viewed by others
Main
Metastatic clear cell renal cell carcinoma (ccRCC) is a highly angiogenic tumour. Most of the cases harbour a mutation, deletion or methylation of the von Hippel Lindau gene, leading to overexpression of VEGF. Therefore, ccRCC represent a paradigm for the use of anti-angiogenic treatments targeting the VEGF/VEGFR pathway. Several tyrosine kinase inhibitors (TKIs) have prolonged progression-free survival (PFS) in patients with metastatic disease. Sunitinib, a multi kinase inhibitor targeting VEGF, PDGF, CSF1 receptors, c-KIT, FLT3 and RET, was the first to be approved in the first-line setting, with a median PFS of 11 months. (Motzer et al, 2009). Other targeted therapies have been developed, including TKIs such as axitinib (Motzer et al, 2013) or pazopanib (Escudier et al, 2014), which inhibit VEGFR1, 2, 3, PDGFR, c-KIT and the VEGF-directed humanised monoclonal antibody bevacizumab (used in combination with interferon (IFN)-α) (Escudier et al, 2010), and the mTOR inhibitors everolimus (Motzer et al, 2010) and temsirolimus (Motzer et al, 2008). More recently, cabozantinib, which inhibits VEGFR, c-MET and AXL (Choueiri et al, 2015), and the anti-PD1 immune checkpoint inhibitor nivolumab, were proposed as other alternatives (Motzer et al, 2015). Although these treatments have finally improved the clinical outcome of metastatic ccRCC, efforts are needed to identify the best treatment regimen according to patient profile. The current therapeutic practices are based on two assumptions: (i) the treatment must destroy blood vessels to eliminate the tumours and (ii) endothelial cells are normal cells that cannot adapt to the selection pressure exerted by the anti-angiogenic treatments. However, tumour cells also express receptors targeted by anti-angiogenic drugs and this may contribute to tumour adaptation and relapse. Another unexpected aspect associated with the use of anti-angiogenesis treatments is the heterogeneity of the patients’ response. Some patients are refractory right away and die rapidly, although others have a transient response and then relapse. A minority of patients are responders for a very long period of time (Motzer et al, 2009; Escudier et al, 2010). These results indicate that ccRCC is a heterogeneous disease with variable clinical evolution (Gerlinger et al, 2012). If the treatment targeted only the genetically stable vasculature, a more homogeneous response would be predicted. Therefore, the treatments induced a ‘Darwinian’ adaptation of tumour cells in relation to the microenvironment. This conclusion lead to two observations: (1) the necessity to identify predictive markers of efficacy; (2) the need for the identification of ‘druggable’ targets participating in progression on anti-angiogenic treatments that should be independent of the VEGF/VEGFR axis.
Preclinical studies have suggested that ELR+CXCL chemokines (C-X-C motif Chemokine ligand containing the glutamic acid, leucine arginine (ELR) motif) may represent prognosis markers of survival in ccRCC patients and may constitute relevant therapeutic targets as evidenced in experimental tumours in mice (Grepin et al, 2012; Grepin et al, 2014). As VEGF/VEGFR, the ELR+CXCL cytokine CXCL7 and its specific receptors CXCR1 and CXCR2 are produced by tumour cells, platelets (von Hundelshausen et al, 2007), endothelial and immune cells (neutrophils and macrophages) (Galliera et al, 2012). They are involved in inflammation and angiogenesis. This generates simultaneously autocrine and paracrine loops that impact the microenvironment.
The current standard of care for ccRCC is to administer anti-angiogenic therapies such as sunitinib. ELR+CXCL chemokines may represent relevant predictive markers for sunitinib efficacy. We investigated whether CXCL7 chemokine easily measured in plasma samples, is a relevant predictive markers of sunitinib efficacy.
Materials and methods
Patients
Eligible patients for SUVEGIL and TORAVA trials were at least 18 years of age and had metastatic ccRCC histologically confirmed, with the presence of measurable disease according to Response Evaluation Criteria in Solid Tumors v1.1. Patients had not received previous systemic therapy for RCC and were eligible for sunitinib or bevacizumab combined with IFN treatment in the first-line setting. Patients were ineligible if they had symptomatic or uncontrolled brain metastases, an estimated lifetime less than 3 months, uncontrolled hypertension or clinically significant cardiovascular events (heart failure, prolongation of the QT interval), history of other primary cancer. All patients gave written informed consent. Tumours were assessed at baseline and then every 12 weeks by thoracic, abdominal, pelvic and bone CT scans. Brain CT scans were performed in case of symptoms.
Study design (SUVEGIL and TORAVA trials)
The prospective cohort includes patients from the SUVEGIL (38 patients) and TORAVA (16 patients) trials.
The SUVEGIL trial (clinicaltrial.gov, NCT00943839) was a multicentre prospective single-arm study. The goal of the trial is to determine whether a link exists between the effectiveness of therapy with sunitinib malate and development of blood biomarkers in patients with kidney cancer. Patients received oral sunitinib (50 mg per day) once daily for 4 weeks (on days 1 to 28), followed by 2 weeks without treatment. Courses repeat every 6 weeks in the absence of disease progression or unacceptable toxicity.
The TORAVA trial (clinicaltrial.gov, NCT00619268) was a randomised prospective study. Patient characteristics and results have been previously described (Negrier et al, 2011). Briefly, patients aged 18 years or older with untreated metastatic ccRCC were randomly assigned (2 : 1 : 1) to receive the combination of bevacizumab (10 mg kg−1 iv every 2 weeks) and temsirolimus (25 mg iv weekly), or the combination of IFN-α (9 mIU iv three times per week) and bevacizumab (10 mg kg−1 iv every 2 weeks), or one of the standard treatments: sunitinib (50 mg per day orally for 4 weeks followed by 2 weeks off) (Negrier et al, 2011).
These studies were approved by the ethic committee at each participating centre and run in agreement with the International Conference on Harmonization of Good Clinical Practice Guideline.
Retrospective validation cohort
Thirty-one patients from the University hospitals of Rennes (France) and Pavia (Italy), treated with sunitinib (50 mg per day, once daily for 4 weeks), were analysed retrospectively.
Efficacy and safety
Blood samples were collected during the inclusion visit (baseline) and at the end of the four weeks of sunitinib administration at each cycle for biochemical analysis.
Plasmatic level of CXCL7 was correlated with OS and PFS, defined respectively as the time from inclusion in the trial to death from all causes (for OS) and to progression, treatment cessation or death (for PFS), censored at last follow-up for those still alive or who have not progressed.
Memorial Sloan-Kettering Cancer Centre (MSKCC/Motzer) score
Memorial Sloan-Kettering Cancer Centre score predicts survival based on clinical and laboratory data in metastatic RCC patients. It is a combination of: (1) performance status (Karnofsky score)<80%, (2) time from diagnosis to systemic treatment<12 months, (3) haemoglobin less than the lower limit of normal, (4) lactate dehydrogenase>1.5 × upper limit of normal, (5) corrected calcium>10 mg dl−1 (2.5 mmol l−1). Score 0, 1–2 or ⩾3 corresponding respectively to Good, Intermediate or Bad Risk Group (Motzer et al, 1999).
Biochemical analysis
Blood samples were centrifuged (10 000 g for 10 min) and the plasmas were collected and conserved at −80 °C. Plasmatic level of CXCL7 (1/100 dilution) was determined by ELISA using Peprotech kits (reference 900-K40).
Quantitative real-time PCR experiments
One microgram of total RNA was used for the reverse transcription, using the QuantiTect Reverse Transcription kit (QIAGEN, Hilden, Germany), with blend of oligo (dT) and random primers to prime first-strand synthesis. SYBR master mix plus (Eurogentec, Liege, Belgium) was used for quantitative real-time PCR (qPCR). The oligonucleotides used for qPCR experiments are described in Supplementary Table S2.
Statistical analysis
Progression-free survival was defined as the time between blood sample collection and progression, or death from any cause, censoring those alive and progression free at last follow-up. OS was defined as the time from blood sample collection to the date of death from any cause, censoring those alive at last follow-up. The CXCL7 cut-off point (250 ng ml−1) for PFS was determined using spline curves analysis. T-test was applied to compare continuous variables and chi-square test, or Fisher’s exact test (when application condition of χ2-test were not fulfilled), were used for categorical variables. Kaplan–Meier method was used to produce survival curves and analyses of censored data were performed using log-rank test. To guarantee the independence of CXCL7 as a predictive factor from validate predictive factor, multivariate analysis were performed using cox regression adjusted on MSKCC score. Adjusted hazard ratio (HR) and 95% confidence interval (95% CI) were calculated.
Smoothing splines curves for HR were used to determined cut-off for censored data.
The predictive cut-off determined in the prospective cohort was validated by using same cut-off, same definition for PFS and OS, and same statistical models in data from retrospective cohort. All analyses were performed using R software, version 3.2.2 (Vienna, Austria, https://www.r-project.org/).
Healthy donor plasma
Plasmas were obtained from healthy donors with informed consent following the Declaration of Helsinki according to recommendations of an independent scientific review board.
Results
Patient and pathological parameters
Fifty-four patients were prospectively enrolled in the SUVEGIL (clinicaltrial.gov, NCT00943839) and TORAVA (clinicaltrial.gov, NCT00619268) trials, and treated by sunitinib. At diagnosis, the median age was 62.96 years. All patients were nephrectomised. Twenty-one patients (38%) had metastasis at diagnosis. Twelve (22%) patients had Fuhrman grade 1–2 and 35 (65%) patients had Fuhrman grade 3–4 tumours. The time from diagnosis to apparition of metastasis was inferior to 1 year for 27 patients (50%) and superior to 1 year for 27 patients (50%). Forty-one of 54 patients were evaluated for the MSKCC score. Eighteen patients (43.9%) had a good MSKCC score, 15 patients (36.59%) had intermediate MSKCC score and 8 patients (19.51%) had a poor MSKCC score. Forty-five patients were prospectively enrolled in the TORAVA clinical trial and treated by bevacizumab in combination with IFN-α or temsirolimus. Forty-two of 45 patients (93.3%) were evaluated for the MSKCC score. Six patients (14.29%) had a good, 13 (30.95%) had and intermediate and 23 (54.76%) had a bad MSKCC score. The population characteristics and pathological parameters are summarised in Table 1.
Thirty-one patients from the University hospitals of Rennes (France) and Pavia (Italy) were included in a validation retrospective cohort. Seven patients (22.58%) had a good, 5 patients (16.13%) had an intermediate and 19 patients (61.29%) had a bad MSKCC score. The patients’ characteristics are also summarised in Table 1.
Progression-free survival in prospective/validation cohorts (sunitinib group) and correlation to CXCL7 plasmatic level
Plasmatic level of CXCL7 was correlated with OS and PFS, defined respectively as the time from inclusion in the trial to death from all causes (for OS) and to progression, treatment cessation or death (for PFS), censored at last follow-up for those still alive or who have not progressed.
The median PFS was 20.5 months and the median OS was 38.4 months (Supplementary Figure S1). The plasmatic level of CXCL7 was measured at diagnosis and throughout the different cycles of sunitinib treatments. No correlation was observed between disease progression and the variation in cytokine plasmatic levels along time. Only plasmatic levels at diagnosis were correlated to PFS. The CXCL7 cut-off point (250 ng ml−1) for PFS was determined using spline curves analysis (R software, version 3.2.2).
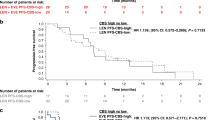
Patients with CXCL7 plasma levels below 250 ng ml−1 (range 139.2–250 ng ml−1) had a shorter median PFS (12.6 months) compared with patients with plasma levels above 250 ng ml−1 (range 250–485.2 ng ml−1, 27.7 months, P=0.001; HR 0.285 (CI 95% 0.161–0.504)) (Figure 1A).
Relationship between plasmatic levels of CXCL7 and PFS of ccRCC patients treated with sunitinib in prospective and retrospective cohort. (A and B) Kaplan–Meier analysis of PFS of patients with ccRCC treated with sunitinib. Progression-free survival (PFS) was calculated from patient subgroups with plasmatic level for CXCL7 at the diagnosis that were less or greater than a cut-off value of 250 ng ml−1, for SUVEGIL and TORAVA trials—prospective analysis (A) or for retrospective analysis (B). Statistical significance (P value) and the time of the median disease free are indicated.
The levels of CXCL7 (cut-off: 250 ng ml−1) were also correlated with OS. Indeed, patients with plasma levels below 250 ng ml−1 had a lower median OS (23.5 months) compared with patients with plasmatic levels above 250 ng ml−1 (not reached, P=0.047; HR 0.374 (CI 95% 0.177–0.79)) (Supplementary Figure S3A).
These results were confirmed in a validation retrospective cohort. Indeed, patients with plasma levels below 250 ng ml−1 had a shorter median PFS (10.4 vs 14.1 months, P=0.0002; HR 0.207 (CI 95% 0.371–0.313)) (Figure 1B).
To guaranty the independence of our biological parameter, it was important to show that CXCL7 was not a surrogate marker of clinical parameters. As shown in Supplementary Table S1, the levels of CXCL7 (inferior or superior at 250 ng ml−1) and clinical parameters of patients in the prospective cohort (age, gender, Fuhrman grade, pT, pN, pM or MSKCC score) are not correlated. The biological and clinical parameters (levels of CXCL7 and MSKCC scores, the standard score used for patient evaluation in clinical practices) were then analysed in a multivariate Cox regression model on PFS (Table 2). CXCL7 expression was identified as an independent prognostic parameter for PFS (P=0.03, HR 0.341 (CI 95% 0.126–0.926)). A similar results was obtained for a bad MSKCC score with respect to PFS (P=0.04, HR 3.13 (CI 95% 0.993–9.865), Table 2).
Progression-free survival in prospective cohort (bevacizumab+IFN group) and correlation to CXCL7 plasmatic levels
To determine the predictive role of CXCL7 for sunitinib efficacy, we tested the plasmatic levels of CXCL7 in a subset of patients of the TORAVA clinical trial that were treated with bevacizumab+IFN. The median PFS was 10.6 months and the median OS was 24.1 months (Supplementary Figure S2). The CXCL7 plasmatic levels did not discriminate patients with a long or a short PFS (Figure 2A) or OS (Supplementary Figure 3B).
Relationship between plasmatic levels of CXCL7 and PFS of ccRCC patients treated with bevacizumab+INF in prospective cohort. Kaplan–Meier analysis of PFS of patients with ccRCC treated with bevacizumab+INF. Progression-free survival (PFS) was calculated from patient subgroups with plasmatic level for CXCL7 at the diagnosis that were less or greater than a cut-off value of 250 ng ml−1, for TORAVA trial—prospective analysis. Statistical significance (P value) and the time of the median disease free are indicated. NR=not reached.
Additional exploratory analyses
Post-hoc analysis was conducted to better define the role of CXCL7 in the tumour.
In silico available transcriptomic data showed that high CXCL7 mRNA levels correlated with tumour stage (Supplementary Figure S4A) and PFS (Supplementary Figure S4B). We compared the level of CXCL7 in the plasma of 24 healthy donors and 37 metastatic ccRCC patients (following surgical removal of the primary tumour). The level of CXCL7 is lower in ccRCC patients (Figure 3A). Moreover, patients that relapse on sunitinib have decreased plasmatic CXCL7 levels compared to responsive patients. Their CXCL7 plasmatic concentrations are not significantly different as compared to healthy donors (Figure 3B).
Correlation between tumour (mRNA) and plasmatic (protein) CXCL7 levels. (A and B) The plasmatic levels of CXCL7 in healthy donors or ccRCC patients were determined by ELISA. (C) The plasmatic and tumour levels of CXCL7 were determined respectively by ELISA and by qPCR. The CC between the two values was calculated. (D) The intra-tumour CXCL7 mRNA levels (7 ccRCC patients), neutrophils (N, LCN2 and ELANE mRNA), M1 macrophages (M1, iNOS and IL1β mRNA) and M2 macrophages (M2, ARG1 and MRC1 mRNA) were determined by qPCR. The CC between each value is indicated. (E) Recapitulative schema: during tumour initiation, the amount of CXCL7-producing cells (neutrophils and M1 macrophages) is more important in the blood stream than in the tumour. Hence, CXCL7 amounts are greater in the blood than in the tumour. Then, the anti-tumour response is linked to an attraction of monocytes to the tumour were they polarised towards M1 macrophages. As tumour cells also express CXCL7, neutrophils are attracted to the tumours. During the tumour development phase, monocytes are polarised towards M2 macrophages that produced CXCL7 in addition to those produced by tumour cells. Attraction of CXCL7-producing cells to the tumour creates an exhaustion of the cytokines in the plasma and an overproduction in the tumour. *P<0.05.
The above-mentioned results were apparently discordant (high intra-tumour levels and low plasmatic levels both correlated with poor prognosis). The intra-tumour CXCL7 mRNA amounts and the plasmatic CXCL7 levels are inversely correlated (correlation coefficient (CC) −0.79, Figure 3C). Physiologically, CXCL7 are produced by platelets (von Hundelshausen et al, 2007) and immune cells (neutrophils and macrophages) (Galliera et al, 2012), and are involved in inflammation and angiogenesis. Hence, we analysed the correlation between intra-tumour CXCL7 amounts and the amounts of immune cells. Intra-tumour CXCL7 is positively correlated with neutrophils (N, CC=0.8) and M2 macrophages invasion (M2, CC=0.85; Figure 3D). Colonisation of ccRCC by these cells has previously been associated with a poor prognosis (Santoni et al, 2014; Song et al, 2015).
To explain the sequence of events during tumour development, we tested plasmatic and intra-tumour CXCL7 levels in experimental tumours of mice xenografted with human ccRCC cells. The experimental tumours obtained by xenografting ccRCC cells developed as we previously described (Grepin et al, 2014; Dufies et al, 2017) (Supplementary Figure S5A). Mouse CXCL7 plasmatic levels were decreased in mice with ‘human’ tumours (Supplementary Figure S5B), which was consistent with the results obtained with plasma from patients. HES staining of the experimental tumours showed an important infiltration of immune cells identified as natural killer (NK), macrophages and neutrophils (Supplementary Figure S5C). A strong correlation was observed in these experimental tumours between human CXCL7 (H CXCL7) and mouse intra-tumour NK cells (CC=0.71) suggesting an immune response triggered by CXCL7. A strong correlation was also observed between mouse CXCL7 (M CXCL7) and the presence of neutrophils (N, CC=0.81). CXCL7 was not correlated with M1 but was correlated with M2 macrophages (M2, CC=0.78), which is consistent with the correlation between N and M2 (CC=0.9). Human and mouse CXCL7 were also correlated with high levels of CXCR1 (CC=0.57 and 0.9 respectively) and CXCR2 (CC=0.85 and 0.55 respectively) which are physiologically expressed on neutrophils and macrophages (correlation between CXCR1 and N, CC=0.6 and correlation between CXCR1 and M2, CC=0.58). A correlation was observed between CXCR2 and NK cells even though NK cells do not express this receptor physiologically (Supplementary Figure S5D).
These results suggest that immune cells producing CXCL7 in the plasma are attracted towards the tumours and participate in the pro-inflammatory/pro-proliferative response contributing in tumour growth (see the schematic representation in Figure 3E).
Discussion
Despite the development of several therapies for ccRCC and the increase of PFS, no curative treatment currently exists. Moreover, because of the heterogeneity of the initial tumours and their subsequent metastasis (Gerlinger et al, 2014), the response to the current first line therapy with sunitinib is highly variable. There are two major constraints. The first evident one is related to the improvement of patient survival. The second is economical and related to the high costs of targeted therapies. In order to reconcile the therapeutic and the economic imperatives, robust predictive markers of treatment efficacy have to be identified. To be manageable in the clinical practices, protocols must be easy to transfer to technical platforms of hospitals, must be non-invasive and applicable to small blood or urine samples.
We believe that we have identified such a marker in the frame of prospective multicentre clinical trials (SUVEGIL and TORAVA), the angiogenic and pro-inflammatory cytokine CXCL7. This was corroborated in a retrospective cohort of patients highlighting the relevance of our results. Moreover, CXCL7 was not indicative of PFS for patients treated with bevacizumab+IFN. These results are strongly indicative of the predictive value of CXCL7 for sunitinib efficacy. Whereas sunitinib induces a significant decrease of cell viability in vitro, bevacizumab+INF did not (Supplementary Figure S6). This result may explain, at least in part, the difference in the predictive value of CXCL7 for sunitinib but not for bevacizumab efficacy. The challenge for a relevant marker is to precisely identify a threshold value. A standard value has been determined through the statistical analysis but must be refined especially for patients whose CXCL7 levels are borderline compared with the identified 250 ng ml−1 threshold value.
We were surprised by the very long PFS (27.7 months) and OS (38.4 months) that we recorded in the prospective clinical trials as compared with those described in the pivotal trial that lead to sunitinib approval (PFS=11 months; OS=20.5 months) (Motzer et al, 2007; Motzer et al, 2009). However, some patients respond for a long period of time to sunitinib reaching 4–5 years (Chara et al, 2011). Therefore, the long PFS and OS probably reflect the recruitment by serendipity of good responders. In a study that had enrolled 4543 patients, a good MSKCC score was correlated to a long PFS (15 months) and a long OS (54.6 months) (Gore et al, 2015). Therefore, these results show in an independent cohort that some patients benefit for a long period of time of sunitinib. However, the important message is the identification of a biological marker that allows identification of very good responders and subsequently patients who benefit of the treatment in terms of PFS and OS.
We were puzzled by the inverse correlation between intra-tumour and plasmatic levels and their relative significance as a prognostic determinant (Grepin et al, 2014). These experiments suggested that during the initial phase of tumour development, immune cells that participate in the physiological plasmatic level of CXCL7 are targeted to the tumour to mount an anti-tumour response, probably via the recruitment of NK cells. However, tumour cells that also produce CXCL7 may participate in immune tolerance, inflammation and angiogenesis via attraction of neutrophils, differentiation of monocytes towards M2 macrophages and proliferation of endothelial cells (neutrophils, monocytes and endothelial cells physiologically expressed CXCL7 receptors). Consequently, CXCL7 levels increase in the tumour whereas they decrease in the plasma (see Figure 3E). Such disequilibrium is probably characteristic of aggressive tumours/metastasis. Such aggressiveness may be attributed to the CXCL7-dependent production of VEGFC and VEGFD, leading to the development of a lymphatic network (Yu et al, 2010), a known feature of metastatic dissemination (Karaman and Detmar, 2014). It would be important to determine whether CXCL7 is a predictive marker of efficacy for other anti-angiogenic drugs targeting the VEGF/VEGFR axis currently used as second or third-line treatments.
Conclusion
Our study highlighted CXCL7 as a relevant predictive marker of sunitinib efficacy, the first line treatment of metastatic ccRCC. We also established a threshold value for patients for low or high risk of relapse in two independent cohorts. These results must be confirmed on a larger prospective cohort to introduce CXCL7 measurements into clinical practice to rapidly adapt the treatment at the diagnosis.
References
Chara L, Rodriguez B, Holgado E, Ramirez N, Fernandez-Ranada I, Mohedano N, Arcediano A, Garcia I, Cassinello J (2011) An unusual metastatic renal cell carcinoma with maintained complete response to sunitinib treatment. Case Rep Oncol 4 (3): 583–586.
Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL, Peltola K, Roth BJ, Bjarnason GA, Geczi L, Keam B, Maroto P, Heng DY, Schmidinger M, Kantoff PW, Borgman-Hagey A, Hessel C, Scheffold C, Schwab GM, Tannir NM, Motzer RJ (2015) Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373 (19): 1814–1823.
Dufies M, Giuliano S, Ambrosetti D, Claren A, Diogop Ndiaye P, Mastri M, Moghrabi W, Cooley LS, Ettaiche M, Chamorey E, Parola J, Vial V, Lupu-Plesu M, Bernhard JC, Ravaud A, Borchiellini D, Ferrero JM, Bikfalvi A, Ebos JM, Khabar KS, Grepin R, Pages G (2017) Sunitinib stimulates expression of VEGFC by tumor cells and promotes lymphangiogenesis in clear cell renal cell carcinomas. Cancer Res 77 (5): 1212–1226.
Escudier B, Bellmunt J, Negrier S, Bajetta E, Melichar B, Bracarda S, Ravaud A, Golding S, Jethwa S, Sneller V (2010) Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 28 (13): 2144–2150.
Escudier B, Porta C, Bono P, Powles T, Eisen T, Sternberg CN, Gschwend JE, De Giorgi U, Parikh O, Hawkins R, Sevin E, Negrier S, Khan S, Diaz J, Redhu S, Mehmud F, Cella D (2014) Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J Clin Oncol 32 (14): 1412–1418.
Galliera E, Corsi MM, Banfi G (2012) Platelet rich plasma therapy: inflammatory molecules involved in tissue healing. J Biol Regul Homeost Agents 26 (2 Suppl 1): 35S–42S.
Gerlinger M, Horswell S, Larkin J, Rowan AJ, Salm MP, Varela I, Fisher R, McGranahan N, Matthews N, Santos CR, Martinez P, Phillimore B, Begum S, Rabinowitz A, Spencer-Dene B, Gulati S, Bates PA, Stamp G, Pickering L, Gore M, Nicol DL, Hazell S, Futreal PA, Stewart A, Swanton C (2014) Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet 46 (3): 225–233.
Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366 (10): 883–892.
Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, Lee SH, Haanen J, Castellano D, Vrdoljak E, Schoffski P, Mainwaring P, Hawkins RE, Crino L, Kim TM, Carteni G, Eberhardt WE, Zhang K, Fly K, Matczak E, Lechuga MJ, Hariharan S, Bukowski R (2015) Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. Br J Cancer 113 (1): 12–19.
Grepin R, Guyot M, Giuliano S, Boncompagni M, Ambrosetti D, Chamorey E, Scoazec JY, Negrier S, Simonnet H, Pages G (2014) The CXCL7/CXCR1/2 axis is a key driver in the growth of clear cell renal cell carcinoma. Cancer Res 74 (3): 873–883.
Grepin R, Guyot M, Jacquin M, Durivault J, Chamorey E, Sudaka A, Serdjebi C, Lacarelle B, Scoazec JY, Negrier S, Simonnet H, Pages G (2012) Acceleration of clear cell renal cell carcinoma growth in mice following bevacizumab/Avastin treatment: the role of CXCL cytokines. Oncogene 31 (13): 1683–1694.
Karaman S, Detmar M (2014) Mechanisms of lymphatic metastasis. J Clin Invest 124 (3): 922–928.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373 (19): 1803–1813.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Kay A, Ravaud A (2010) Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 116 (18): 4256–4265.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372 (9637): 449–456.
Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J, Hariharan S, Chen C, Rosbrook B, Kim S, Rini BI (2013) Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 14 (6): 552–562.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27 (22): 3584–3590.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356 (2): 115–124.
Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J (1999) Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 17 (8): 2530–2540.
Negrier S, Gravis G, Perol D, Chevreau C, Delva R, Bay JO, Blanc E, Ferlay C, Geoffrois L, Rolland F, Legouffe E, Sevin E, Laguerre B, Escudier B (2011) Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol 12 (7): 673–680.
Santoni M, Berardi R, Amantini C, Burattini L, Santini D, Santoni G, Cascinu S (2014) Role of natural and adaptive immunity in renal cell carcinoma response to VEGFR-TKIs and mTOR inhibitor. Int J Cancer 134 (12): 2772–2777.
Song W, Yeh CR, He D, Wang Y, Xie H, Pang ST, Chang LS, Li L, Yeh S (2015) Infiltrating neutrophils promote renal cell carcinoma progression via VEGFa/HIF2alpha and estrogen receptor beta signals. Oncotarget 6 (22): 19290–19304.
von Hundelshausen P, Petersen F, Brandt E (2007) Platelet-derived chemokines in vascular biology. Thromb Haemost 97 (5): 704–713.
Yu M, Berk R, Kosir MA (2010) CXCL7-mediated stimulation of lymphangiogenic factors VEGF-C, VEGF-D in human breast cancer cells. J Oncol 2010: 939407.
Acknowledgements
This work was supported by the French association for cancer research (ARC), IRIS Pharma (PDN), the Fondation de France (SG and MD financial supports), the French National Institute for Cancer Research (INCA), Foundation François Xavier Mora (SG financial support), the Région Provence Alpes Cote d’Azur (PND financial support) and the Fondation Cordon de Vie Monaco. We thank the IRCAN core facilities (animal facility) for technical help. We also thank the Department of Pathology, especially Arnaud Borderie and Sandrine Destree, for technical help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Dufies, M., Giuliano, S., Viotti, J. et al. CXCL7 is a predictive marker of sunitinib efficacy in clear cell renal cell carcinomas. Br J Cancer 117, 947–953 (2017). https://doi.org/10.1038/bjc.2017.276
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2017.276
Keywords
This article is cited by
-
Frontier knowledge and future directions of programmed cell death in clear cell renal cell carcinoma
Cell Death Discovery (2024)
-
Unveiling CXCR2 as a promising therapeutic target in renal cell carcinoma: exploring the immunotherapeutic paradigm shift through its inhibition by RCT001
Journal of Experimental & Clinical Cancer Research (2024)
-
The tyrosine-kinase inhibitor sunitinib targets vascular endothelial (VE)-cadherin: a marker of response to antitumoural treatment in metastatic renal cell carcinoma
British Journal of Cancer (2018)