Abstract
Background:
Few epidemiological studies have prospectively investigated preoperative and surgical risk factors for acute postoperative pain after surgery for breast cancer. We investigated demographic, psychological, pain-related and surgical risk factors in women undergoing resectional surgery for breast cancer.
Methods:
Primary outcomes were pain severity, at rest (PAR) and movement-evoked pain (MEP), in the first postoperative week.
Results:
In 338 women undergoing surgery, those with chronic preoperative pain were three times more likely to report moderate to severe MEP after breast cancer surgery (OR 3.18, 95% CI 1.45–6.99). Increased psychological ‘robustness’, a composite variable representing positive affect and dispositional optimism, was associated with lower intensity acute postoperative PAR (OR 0.63, 95% CI 0.48–0.82) and MEP (OR 0.71, 95% CI 0.54–0.93). Sentinel lymph node biopsy (SLNB) and intraoperative nerve division were associated with reduced postoperative pain. No relationship was found between preoperative neuropathic pain and acute pain outcomes; altered sensations and numbness postoperatively were more common after axillary sample or clearance compared with SLNB.
Conclusion:
Chronic preoperative pain, axillary surgery and psychological robustness significantly predicted acute pain outcomes after surgery for breast cancer. Preoperative identification and targeted intervention of subgroups at risk could enhance the recovery trajectory in cancer survivors.
Similar content being viewed by others
Main
Breast cancer is the commonest cancer affecting women with a 5-year survival above 80% as a result of earlier diagnosis and improved management (UK, 2011). Understanding and monitoring the acute and long-term sequelae of treatment is an increasingly high priority (Harrington et al, 2010; Ganz et al, 2011; Richards et al, 2011). Painful adverse symptoms can last for many years after surgery for breast cancer and the adverse impact upon postoperative quality of life is well documented (Macdonald et al, 2005; Gärtner et al, 2009; Peuckmann et al, 2009; Andersen and Kehlet, 2011). However, less is known about who is at greatest risk of developing adverse postoperative outcomes after cancer surgery. Improving the identification and typology of risk subgroups should provide opportunities for targeting prevention and treatment initiatives in cancer survivors.
Severe pain in the acute postoperative period has consistently been found to predict chronic postsurgical pain, although few studies have prospectively examined whether this relationship exists after surgery for breast cancer (Katz et al, 2005). One systematic review of 32 studies identified preoperative pain, anxiety, age and type of surgery as significant predictors of acute postoperative pain after different types of surgery, although only two studies involving breast surgery were included, neither of which found any relationship with preoperative pain (Ip et al, 2009). Methodological quality of studies was poor, hampered by small sample sizes, or failure to capture important preoperative pain and psychological variables.
Postsurgical pain is known to be predominantly neuropathic in character (Bruce et al, 2004), whereby neuropathic pain is defined as ‘pain caused by a lesion or disease of the somatosensory system’ (Jensen et al, 2011). However, few studies record detailed information on the chronicity or character of pain experienced by patients before breast cancer surgery. This is important as postoperative pain may be a continuation of pre-existing pain rather than an incident adverse event attributable to surgery. Chronic preoperative pain may contribute to central sensitisation from longstanding exposure to nociceptive input (Wilder-Smith et al, 2002). Preoperative pain may be amplified or accelerated by surgical incision, subsequent tissue injury, pathophysiological responses and local inflammatory processes (Kehlet et al, 2006; Gerbershagen et al, 2010). Surgical factors contribute to the genesis of pain: intraoperative nerve damage may contribute to the severity, character and chronicity of postoperative pain. Preservation of the intercostobrachial nerve (ICBN) may be difficult because of anatomical location, traversing the axilla in close relation to the axillary lymph nodes (Loukas et al, 2006). The relatively new surgical technique of sentinel lymph node biopsy (SLNB) avoids unnecessary axillary dissection in many patients, thereby reducing postoperative arm morbidity (Fleissig et al, 2006).
Acute postoperative pain severity is predicted by emotional distress, particularly anxiety and depression (Johnston, 1986; Johnston and Vögele, 1993; Katz et al, 2005). Most current literature focuses upon psychological vulnerability factors or absence of vulnerability predicting postoperative outcome, rather than specifically investigating the role of psychological robustness or resilience and whether these could be protective against adverse outcomes. Optimism, considered a personality trait, has been broadly conceptualised as optimists attributing negative events to external factors, whereas pessimists attribute negative events to internal factors and generally considering these as persistent and stable rather than temporary (Peterson et al, 1982; Meevissen et al, 2011). Dispositional optimism has been associated with coping strategies relevant to postoperative recovery in patients undergoing coronary artery bypass surgery (Scheier et al, 1989). The degree to which potentially protective factors such as positive disposition (e.g., positive affect, optimism) and other psychological vulnerability factors (e.g., pain catastrophizing or negative affect) and are associated with acute pain after breast cancer surgery is currently unknown.
Recent recommendations call for large-scale prospective studies incorporating repeated longitudinal assessment to investigate predictors of surgical outcomes (Katz, 2011; Kehlet and Dahl, 2011), and improved postoperative pain reporting (Srikandarajah and Gilron, 2011). Empirical determination of antecedent preoperative, intraoperative risk and protective factors for acute postoperative pain will inform our understanding of transitional pathways towards pain chronicity (Katz, 2011). Moreover, it will enhance our understanding of the burden of pain-related adverse events in cancer survivors (Harrington et al, 2010).
This study investigated whether preoperative psychological vulnerability (anxiety, depression, pain catastrophizing, surgical worry) and psychological ‘robustness’ (positive affect, dispositional optimism), pain status and surgical factors were associated with acute pain severity, in the first week after breast and axillary surgery. We hypothesised that psychological distress, adjusted for demographic and clinical factors including intraoperative nerve dissection, would predict higher acute pain severity (pain at rest (PAR); movement-evoked pain), while psychological robustness would be protective against acute pain outcomes after breast and axillary surgery. Furthermore, we hypothesised that chronicity of preoperative pain would be associated with increased severity of acute postoperative pain.
Materials and methods
Design, setting and inclusion eligibility
The Study of Recovery after Breast Surgery (Recovery Study) is an epidemiological, prospective cohort study that recruited women from four breast cancer units, serving a catchment population of 574 027 (females, all ages), across the North of Scotland. Newly diagnosed women aged ⩾18 years, with histologically proven primary invasive or non-invasive breast cancer, requiring surgical excision of their tumour with or without axillary surgery, were eligible for inclusion. Exclusion criteria included: men, women aged <18 years, pregnant women, history of major psychiatric disorder, previous breast or axillary surgery, bilateral surgery, recurrent disease or detectable metastatic disease at the time of initial diagnosis. Surgery was the first line of treatment, therefore other clinical variables relating to adjuvant therapies, chemotherapy and/or radiotherapy are not reported in this manuscript.
Participant recruitment
Recruitment and consent was undertaken following diagnosis at breast clinics and screening centres, or on the hospital ward when patients were admitted before surgery. Clinical or research staff invited patients to participate and provided packs containing an information sheet, consent form and baseline questionnaire. Participants provided signed consent for access to medical records for research purposes. The study was approved by Fife and Forth Valley National Health Service (NHS) Multicentre Research Ethics Committee with local governance approvals obtained from separate regional NHS organisations.
Sample size
We used national breast cancer incidence data to estimate regional annual new diagnoses for Northern Scotland, calculating that 675 women would be eligible for invitation to participate over an 18-month period. The study was powered to detect chronic postsurgical pain at longer-term follow-up: we estimated a pain incidence of 43% at 9 months after surgery (Smith et al, 1999). We assumed a 60% response rate from 675 women and 70% response rate at 9 months (n=405). A recruited sample of 405 women would provide 80% power to detect a 3.4 unit difference in psychological distress measured using the State Trait Anxiety Inventory (STAI) between those with and without chronic pain, assuming a s.d. of 10 units (Tasmuth et al, 1996).
Data collection
Questionnaires and data collection tools were modelled on our previous quantitative studies of chronic pain (Smith et al, 1999; Bruce et al, 2003; Macdonald et al, 2005; Powell et al, 2012). Demographic variables included age, marital status, highest qualification achieved and employment status reported in preoperative questionnaires. Body mass index was calculated from height and weight measured on admission for surgery. Women were allocated to a Scottish Index of Multiple Deprivation quintile using Scottish postcode data (1 most deprived, 5 most affluent).
Preoperative pain
Detailed assessment of the character, location and duration of any pain was undertaken to determine ‘pain history’ before surgery. The International Association for the Study of Pain definition of continuous or intermittent pain lasting for 3 months or longer was used to define chronic preoperative pain (IASP, 1994). Patients reporting any ache, pain, discomfort, altered sensations or numbness in the previous week were asked to provide further information using upper body maps, pain-related symptom grids and the following validated neuropathic pain instruments: Self-completed Leeds Assessment of Neuropathic Symptoms and Signs pain scale (S-LANSS) (Bennett et al, 2005; Bouhassira and Attal, 2009), and the ‘Douleur Neuropathique 4’ (DN4) questionnaire (Bouhassira et al, 2005). The S-LANSS and DN4 have been used in epidemiological surveys, whereby scores of ⩾12 and ⩾3, respectively, are indicative of pain with neuropathic characteristics. Self-reported co-morbidity, from a predetermined list of conditions, was categorised as painful or non-painful comorbidity. This list included 15 conditions, of which 10 were considered to be ‘painful’ (e.g., migraine, angina, back problems and so on). We used the Functional Assessment of Cancer Therapy-Breast questionnaire arm subscale to assess preoperative arm morbidity: swelling/tenderness, numbness, painful movement, range of movement and stiffness. Lower scores indicate greater arm morbidity (range 0–20).
Psychological variables
A range of emotional and cognitive variables were measured preoperatively to capture psychological status. Full standardised instruments were used to measure psychological vulnerability (anxiety, depression, catastrophizing and surgical worry); and robustness (positive affect and dispositional optimism). The STAI was used to measure state and trait anxiety, whereby higher scores indicate greater anxiety (range 20–80) (Spielberger et al, 1983). Depressive thoughts were assessed using the Hospital Anxiety and Depression Scale (HADS) depression subscale, with higher scores indicating poorer mental health (range 0–21) (Zigmond and Snaith, 1983). The 13-item Pain Catastrophizing Scale (PCS) was used to capture pain catastrophizing, defined as an exaggerated negative orientation to aversive stimuli (Pavlin et al, 2005). Total PCS scores range from 0–52 with higher values indicating greater catastrophizing. Surgical worry was captured using a single item asking patients to rate ‘how worried you are about your operation’ (four-category response) modified from previous studies (Broadbent et al, 2003; Powell et al, 2012). The full Positive and Negative Affect Scale (PANAS) was used to capture affect; the timing of the stem question ‘how you generally feel’ was applied, with higher scores indicating greater positive affect (range 10–50) (Watson et al, 1988). Two indicators of psychological robustness were assessed: the tendency to experience general positive affect was captured using the Positive Affectivity scale of the PANAS (PANAS-PA). Dispositional optimism, defined as generalised outcome expectancies that good things, rather than bad things will happen, was measured using the short version of the Life Orientation Test (LOT) (Scheier and Carver, 1987) (scale range 0–32).
Clinical/surgical variables
Breast surgery was categorised as wide local excision (WLE), mastectomy or mastectomy with immediate reconstruction. Axillary procedures were categorised as SLNB, axillary four node sample (ANS) or axillary node clearance (ANC). Data on tumour grade and status were extracted from medical records. Surgeons were asked to record whether or not the ICBN was identified, and, if identified, whether the nerve was preserved with no apparent damage, preserved with potential damage, the main trunk was divided or some branches divided and others preserved at the time of surgery.
Anaesthetic variables
A pragmatic, open protocol was permitted for anaesthetic regimes. General anaesthesia was induced with propofol and fentanyl or alfentanil with volatile maintenance using isoflurane, sevoflurane or desflurane together with nitrous oxide or air. Intraoperative morphine up to 10 mg intravenous was used for mastectomy or axillary clearance, with bupivacaine infiltration of the breast around the site of skin incision used for WLE’s at the end of surgery. Bupivacaine infiltration was also administered to the axillary wound following ANS or SLNB. Usual analgesia included intravenous paracetamol (1 g) and 10 mg or 30 mg IV ketorolac, dependent upon age and comorbidity. Postoperative analgesia was 1 g paracetamol 6–8 hourly as required.
Outcome variables
After surgery, patients were contacted by telephone on the 7th postoperative day and asked to report PAR and movement-evoked pain (MEP), on average in the first week and in the preceding 24 hours, using a numerical rating scale (NRS) from 0 (no pain) to 10 (worst pain imaginable) (Cleeland and Ryan, 1994). Women were asked about pain on movement rather than a specific movement or activity. Data were collected on number of wounds, presence of drain, which wound was (most) painful, pain severity and analgesic consumption. Pain character was assessed by asking women to select the ‘best’ descriptor for their most painful wound or related-area. Pain descriptors offered included: ache, pain, discomfort, altered sensations or numbness. These descriptors were selected from the literature and from our previous research with women reporting chronic pain after breast cancer surgery (Smith et al, 1999; Macdonald et al, 2005; Baron et al, 2007). Women were asked whether they had taken pain killers during the week since the operation and in the last 24 hours.
Statistical analysis
Descriptive analyses were undertaken on preoperative, surgical and acute pain data. Categorical data were described using frequencies and percentages, continuous data using mean (s.d.) for normally distributed data and median (interquartile range) for skewed data. Cronbach’s alpha was used to measure the internal consistency of psychological subscales. First, univariate analyses were used to investigate associations between preoperative characteristics and acute postoperative pain. Pain at rest and MEP in the first postoperative week were studied using a threshold of ⩾4 on the 0–10 NRS (Peters et al, 2007; Gärtner et al, 2009; Srikandarajah and Gilron, 2011). Chi-squared tests (with continuity correction) were used for categorical variables, independent t-tests for normally distributed continuous variables and Mann–Whitney tests for skewed continuous variables.
Multiple logistic regression models were then used to determine whether selected preoperative psychological variables (vulnerability: STAI state anxiety, STAI trait anxiety, HADS depression, PCS and surgical worry; robustness: PANAS-PA and LOT) were associated with acute PAR and MEP after adjusting for age and clinical variables (type of breast surgery, type of axillary surgery, ICBN status, preoperative chronic pain). The list of variables to be included in the models was prespecified by the Recovery Study Group. As there was evidence of multicollinearity between the psychological variables, factor analysis was used to reduce these to a smaller number of factors. An approach using principal components with promax rotation was used and the number of included factors decided after consideration of the eigenvalues and the slope of the scree plot. The included factors were then incorporated within the multiple logistic regression models predicting PAR and MEP, controlling for age and the clinical variables.
Results
Response rate
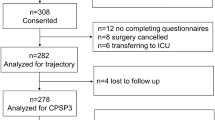
A total of 406 women were recruited from four breast cancer units; of these, 44 women (10.8%) were excluded from the study (preoperative questionnaire completed postoperatively n=26; previous breast cancer surgery n=9; bilateral breast procedure n=4, major psychiatric disorder n=2; other n=3). Preoperative data were available for 362 women. Acute pain data were obtained from 341 (94%), but three women were excluded from the acute pain analyses as their data were collected more than 30 days after surgery (Figure 1). The study population with complete data therefore comprises 338 women. Median (IQR) time from completion of baseline questionnaire to surgery was 1 day (1–4 days); 90% of women underwent surgery within 2 weeks of completion of baseline questionnaire. Median (IQR) time from surgery to the acute pain assessment was 8 (7–10) days. Preoperative demographic, surgical and psychological characteristics for the full sample are presented in Table 1.
Sample characteristics
Mean age was 59.1 years (s.d. 10.8); most women were married (241/362, 67%) or widowed (45/362, 12%). Almost half of the sample were retired (162/362, 45%) with most of the remainder working full-time, part-time or being self-employed (160/362, 44%). A high proportion of women had completed work-related (64/360, 18%), college (116/360, 32%) or degree, qualifications (71/360, 20%).
Preoperative pain character
Overall, 151/359 (42%) women reported ache, pain, discomfort, altered sensations or numbness in the upper body in the week before surgery; of these only 56/359 women (16%) fulfilled the definition of chronic preoperative pain, with painful symptoms in the upper body persisting for 3 months or more before surgery. Neuropathic pain scores were obtained for those reporting any preoperative pain (including ache, discomfort, altered sensations, numbness) in the upper body (n=151): mean DN4 scores were 1.32 (s.d. 1.5; n=134); mean S-LANSS scores were 5.30 (s.d. 5.5; n=148) (Table 1). The overall proportion of women categorised as having preoperative pain of predominantly neuropathic origin was low: 27/359 (8%) were DN4-positive and 22/359 (6%) were S-LANSS-positive.
Surgical variables
Overall, 228/357 women underwent WLE (64%), 92 (26%) underwent mastectomy, with 15 (4%) women undergoing mastectomy with concurrent reconstructive surgery. The most frequently performed axillary procedure was SLNB (146/347, 42%) followed by ANC (107/347, 31%) and ANS (94/347, 28%). Most tumours were invasive breast cancers (342/359, 95%) rather than non-invasive (in situ cancer) (17/359, 5%), with tumour grade 1 (45/359, 13%), 2 (183, 51%) or 3 (131 37%) and with axillary node status graded as negative (243/356, 68%) or positive (113/356; 32%). Complete data on ICBN handling was obtained for 350/362 women (97%); the ICBN was divided or damaged in 110/351(31%).
Acute postoperative pain
In the first postoperative week, 308/336 (94%) women reported any PAR (NRS⩾1). Mean (s.d.) pain scores at rest in the first postoperative week were: PAR 3.18 (2.13) (N=336) and MEP 3.83 (2.32) (N=335). On the 7th postoperative day, mean (s.d.) scores for PAR were 2.76 (2.10) (N=336) and 3.33 (2.24) for MEP (N=336). Moderate to severe PAR (⩾4 on 0–10 NRS) was reported by 137/336 (40.8%) women, with 169/335 (50.4%) reporting moderate to severe MEP during the first postoperative week. On the day of assessment, 182/336 (54.2%) had taken analgesics in the previous 24 h.
Postoperative pain descriptor and location
The most commonly reported pain-related descriptor in the first week after surgery was ‘discomfort’ (141/338; 42%), followed by ‘altered sensations’ (85/338; 25%), ‘pain’ (85/338; 25%) and ‘numbness’ (83/338; 25%). Only 27/338 (8%) women chose ‘ache’ and 31/338 (9%) women did not choose any descriptor. Women were less likely to experience altered sensations or numbness after SLNB compared with ANS or ANC surgery (Table 2). The likelihood of experiencing postoperative numbness and altered sensation increased with the extent of axillary surgery: sentinel biopsy (50/137, 36%), axillary sampling (36/88, 41%), axillary clearance (51/103, 50%) (P=0.045, χ2 test for trend).
Preoperative predictors of moderate to severe acute postoperative pain
Univariate analyses examined whether preoperative and perioperative factors were associated with moderate to severe PAR and MEP in the first postoperative week, using a threshold of ⩾4 on the 0–10 NRS (Table 3). Greater preoperative arm morbidity, higher levels of state and trait anxiety, depression, lower optimism and greater surgical worry were associated with moderate to severe (⩾4 on 0–10 NRS) acute postoperative PAR and MEP in the first postoperative week. There was evidence that higher preoperative pain catastrophizing scores were associated with moderate to severe PAR (Mann–Whitney P=0.03); although this was only of borderline significance for pain on movement (P=0.08). Preoperative chronic pain was a statistically significant predictor of both PAR and MEP (Table 3). A significantly lower proportion of women with ICBN preservation reported moderate to severe acute postoperative MEP (P=0.004). Two logistic regression models were then used to investigate which factors were associated with PAR and MEP ⩾4 in the first postoperative week (results not shown). There was no evidence that any of the psychological variables were associated with either MEP or PAR in these multivariate analyses, but there was a strong suggestion of multicollinearity between these variables: this can cause inflated s.e. and therefore wider than expected confidence intervals.
Factor analysis was used to reduce the psychological variables to a smaller number of factors (Table 4). The first derived component had an eigenvalue of 3.52 and was particularly associated with higher values of PANAS-PA and LOT optimism, also with lower values of STAI trait anxiety and HADS depression. The second component had an eigenvalue of 1.06 and appeared to be more related to current worry (e.g., worry about the operation) but given the relatively low eigenvalue of the second component and the slope of the scree plot, only the first component was selected for the multivariate analysis. This component was labelled ‘psychological robustness’, as it would appear to fit within the broader dimension of resources that characterise psychological positivism; thus combining a ‘habitual style of anticipating favourable outcomes’ (Scheier and Carver, 1987) and reflective of ‘energy, excitement and enthusiasm’ (Watson and Pennebaker, 1989). This component, representing dispositional optimism and ‘psychological robustness’, was then entered into the two logistic regression models predicting PAR and MEP adjusting for age and clinical variables (Table 5). There was strong evidence that preoperative psychological robustness was associated with reduced likelihood of both PAR (OR: 0.63, 95% CI: 0.48–0.82) and MEP (OR: 0.71, 95% CI: 0.54–0.93) in the first week after surgery. Compared with SLNB, patients undergoing axillary node sampling (OR: 2.17, 95% CI: 1.15–4.08) and nodal clearance (OR: 3.45, 95% CI 1.45–8.46) were significantly more likely to experience moderate to severe PAR and MEP in the first postoperative week. Using a 3-category classification for ICBN handling, significantly lower odds of moderate to severe MEP after breast cancer surgery was observed after intraoperative nerve division or damage.
Discussion
We have used an epidemiological study design to identify preoperative demographic, psychological, pain-related and surgical risk factors for moderate to severe acute postoperative pain after surgery for breast cancer. Chronic preoperative pain, type of axillary surgery and psychological ‘robustness’, specifically dispositional optimism and positive affect were statistically significant independent predictors of acute postoperative pain severity in the first week after breast and axillary surgery. There was no evidence of a relationship between age or type of breast surgery and acute pain outcomes. Interestingly, we observed a significantly reduced risk of moderate to severe PAR and MEP after nerve preservation or intraoperative nerve division/damage.
Overall, 41% (137/336) and 50% (169/335) of women reported moderate to severe PAR and MEP, respectively, on the 7th postoperative day after breast cancer surgery. Despite advances in perioperative anaesthesia and analgesia, studies suggest that fewer than half of patients receive adequate pain relief after surgery (Rawal and Allvin, 1998). Our NRS cutoff of ⩾4 to indicate clinically relevant moderate to severe pain is comparable with other literature of breast surgery and other surgical procedures (Peters et al, 2007; Aasvang et al, 2009; Gärtner et al, 2009). Pain scores of ⩾4 have been empirically demonstrated to affect general activity, mood and postoperative mobility after surgery, and are considered clinically important for treatment (Peters et al, 2007).
We hypothesised that increased psychological robustness and greater psychological vulnerability would predict acute pain severity. Although univariate analysis revealed significant associations between all distress-related variables and increased severity of acute pain, these were not statistically significant after adjustment for other variables. Nevertheless, this may be a result of collinearity between the variables. Our derived composite variable, incorporating dispositional optimism and positive affect, was protective against acute postoperative pain. Optimism has been positively associated with a range of health outcomes, including symptom report and other recovery measures after surgery (Scheier and Carver, 1987; Peters et al, 2007). In a heterogeneous sample of surgical patients, not including those with breast cancer, higher levels of dispositional optimism before surgery preoperatively predicted better mental health and vitality at 12-month follow-up (Peters et al, 2010). However, the previous literature mostly focuses upon vulnerability or absence of vulnerability predicting postoperative outcome, rather than specifically investigating the role of psychological robustness. For example, negative affectivity has been found to predict self-reported symptoms in a range of health conditions (Watson and Pennebaker, 1989). Watson and Pennebaker propose that those who are high in negative affectivity are more vigilant of symptoms, thus are more likely to be aware of body sensations such as pain. This is supported by literature arguing that psychological variables can be considered constituent components of negative affectivity. For example, pain may seem less severe to those who are relaxed compared with those who are anxious (Rainville et al, 2005). However, other mechanisms of effect are possible: anxiety or pain catastrophising may affect behaviours that could influence recovery, as per fear avoidance theories of chronic pain (Leeuw et al, 2007). One early surgical study found that dispositional optimism was associated with the coping strategies of making plans and setting goals relevant to postoperative recovery by patients before undergoing coronary artery bypass surgery (Scheier et al, 1989). There is good evidence that psychological preparation for surgery, addressing preoperative emotions and cognitions, improves a range of recovery-related outcomes, including postoperative pain, suggesting there are opportunities for intervention before surgery for breast cancer (Johnston and Vögele, 1993; Powell et al, 2010).
We also examined whether the presence of chronic preoperative pain predicted the severity of acute postoperative pain. There was strong evidence for this, independent of preoperative psychological status and intraoperative nerve dissection: women with chronic preoperative pain were more than three times more likely to report stimulus-dependent MEP (OR 3.19; 95% CI 1.47–6.92). We prospectively assessed prior pain experience before surgery, distinguishing between chronic and non-chronic painful symptoms, to exclude pain associated with preoperative investigations, e.g., diagnostic core biopsy. The mechanisms for pain predicting pain are unclear, although likely to be due to neuroplasticity from sustained alterations to central nervous function, e.g., central neuroplasticity has been demonstrated in patients with chronic low back pain before spinal surgery (Wilder-Smith et al, 2002).
The presence of preoperative pain and comorbidities are associated with increased risk of acute and chronic pain, although evidence for chronic postsurgical pain is predominantly from studies of stump and phantom pain after amputation surgery and from musculoskeletal conditions rather than breast cancer surgery (Wilder-Smith et al, 2002). A Danish national cross-sectional study reported increased risk of chronic pain after breast cancer surgery in those reporting low back pain and headache, although findings were based upon patient recall of preoperative morbidity from 2 years previously (Gärtner et al, 2009).
With regards to surgical variables, axillary sampling and clearance were associated with significantly higher rates of pain in the first week after surgery compared with sentinel node biopsy. Our study included the migration from the four node sampling technique to SLNB, thus provided the opportunity to identify variation in outcome reporting by different axillary surgical techniques. However, It is worth noting that the four node sample technique is now of historical interest (Goldhirsch et al, 2003), although may still be routine in centres with limited access to nuclear medicine. We found an unexpected decrease in the odds of moderate to severe acute postoperative pain after both ICBN preservation or nerve damage or division. This may relate to reporting differences, whereby some surgeons reported ‘nerve not identified’ during WLE and others reported ‘nerve preserved’. However, it may be clinically plausible that patients experience postoperative numbness rather than intense pain immediately after nerve division. The likelihood of experiencing postoperative neuropathic-type characteristics of numbness and altered sensations increased by extent of axillary surgery: sentinel biopsy (37%), axillary sampling (41%) vs axillary clearance (50%) (P<0.05). Whether immediate numbness and altered sensations predict long-term pain outcome will be determined from long-term follow-up of our sample; numbness and paraesthesia in the first postoperative week may mask or dampen painful symptoms in the acute and subacute recovery period. We also cannot exclude the possibility that intensive analgesia during hospitalisation contributes to the response to pain during the first postoperative week. The pathway to pain chronicity is often conceptualised as linear, yet it is plausible that differences in pain descriptors at various recovery time points may reflect distinct underlying pathophysiological mechanisms.
In addition to duration of preoperative pain, our data are novel with respect to exploring pain character before and immediately after breast and axillary surgery. We found no relationship between preoperative neuropathic pain and severity of postoperative pain, although the overall prevalence of neuropathic characteristics before surgery was low (6–7%). Reassuringly, prevalence in our sample is comparable with national epidemiological surveys screening for neuropathic pain (Attal et al, 2011).
Pain is a subjective, patient-reported outcome. We accepted self-report of neuropathic characteristics without clinical examination, laboratory-based electrophysiological testing or brain imaging (e.g. microneurography or functional magnetic resonance imaging. Although these detailed investigations have been recently endorsed within international guidelines for the assessment of neuropathic pain (Haanpää et al, 2011), these methods are largely incompatible with epidemiological, population-based studies. Postoperatively, we enquired about the most painful wound or area, assuming this would correspond to the most troublesome or bothersome pain. Rather than using a pain character questionnaire, we offered women a choice of verbal pain descriptors. Most women were encouraged to select a single ‘best’ descriptor for their pain, but due to differences in data collection, some assessors allowed women to select more than one descriptor. At the time of patient recruitment, there were no published neuropathic screening instruments suitable for administration by telephone, nor had any instruments been validated for the immediate postoperative period. However, our selection of pain descriptors was empirically driven, with terms extracted from our previous quantitative studies and qualitative transcripts from interviews with women reporting persistent postsurgical pain 9–12 years after mastectomy (Macdonald et al, 2005). Until recently, diagnosis of neuropathic pain was based upon deficits identified from sensory examination although recent research has demonstrated good discriminant ability using pain descriptors, including numbness. Consequently, clinical examination may not be mandatory for the diagnosis of neuropathic pain (Bouhassira et al, 2005).
Methodological strengths
This is the first prospective study to identify the role of preoperative psychological, clinical and pain-related predictors of acute postoperative pain after breast and axillary surgery. Our sample was large enough to permit multiple comparisons of putative predictors. The methodological strengths include geographical coverage, whereby patients were recruited from a diverse population from urban, rural and remote-rural locations across Northern Scotland. The sociodemographic distribution of our sample reflected the UK breast cancer incidence data, with a higher age-standardised incidence observed among those with the least socioeconomic deprivation (Shack et al, 2008). This study complies with current demands for larger postsurgical studies with detailed preoperative data collection and comprehensive assessment of pain history (location, character, duration, comorbidity), psychological and physical health before surgery (Kehlet and Rathmell, 2010; Katz, 2011). Few surgical epidemiology studies have attempted to record and adjust for intraoperative nerve handling. Surgeons contributed to the design of ICBN data collection forms, which may account for the high return rate of intraoperative nerve data (97%). Additionally, the precise classification of axillary procedures distinguishes between contemporaneous techniques of sentinel node biopsy, axillary sampling and axillary clearance; other studies of pain outcomes have grouped the different surgical procedures that patients undergo to their axilla together, have used binary comparisons or have excluded axillary procedures completely (Steegers et al, 2008; Fecho et al, 2009; Gärtner et al, 2009).
Limitations
We allowed an open, pragmatic approach to anaesthesia and perioperative analgesia and did not systematically adjust for prevention or treatment modalities, which may impact upon pain reporting. However, an open policy approach has been followed by many other epidemiological and clinical studies of acute and chronic postoperative pain (Peters et al, 2007). The majority of our admissions were short-stay (<48 h), limiting our ability to prospectively track analgesic consumption and pain relief from medication. However, a recent review highlighted that demographic, surgical and psychological factors, rather than anaesthesiology variables, are important for prediction of acute pain severity (Ip et al, 2009). Pain intensity in the initial hours and days whilst hospitalised is masked by analgesic use, thus it has been argued that later acute pain scores are more reflective of overall acute postoperative pain (Gottschalk and Ochroch, 2008). Hence, our selection of day 7 pain outcomes: severe acute pain on postoperative days 4 (Peters et al, 2007), 6 and 7 (Gottschalk and Ochroch, 2008), has been found to predict CPSP.
Conclusions
The Recovery study has generated important data on the clinical and psychological predictors of acute postoperative pain after surgery for breast cancer and has identified potentially modifiable factors that may respond to preoperative intervention. We found strong evidence for surgically induced moderate to severe acute postoperative pain in women with chronic preoperative pain and those having more invasive axillary procedures, and conversely, those who had high levels of dispositional optimism and positive affect were less likely to report postoperative pain. Identification of those most at risk of acute and persistent postoperative complications is important for management and also supporting long-term recovery in these cancer survivors (Ganz et al, 2011). Clinicians and healthcare professionals should, therefore, be aware of increased risk of poor outcome in those with a history of chronic pain. There is potential for evaluation of brief preoperative cognitive behaviour interventions, such as cognitive reframing or other psychological coping techniques, targeted at enhancing positive outlook and expectations before undergoing surgery for breast cancer. Women should be instructed on the importance of pain management, with further exploration to identify whether postoperative analgesic needs are being met. We aim to follow-up of our patient cohort to monitor chronic pain-related outcomes and recovery trajectory after surgery for breast cancer.
Change history
18 August 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Aasvang EK, Hansen JB, Kehlet H (2009) Pre-operative pain and sensory function in groin hernia. Eur J Pain 13 (10): 1018–1022
Andersen KG, Kehlet H (2011) Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain 12 (7): 725–746
Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D (2011) The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain 152 (12): 2836–2843
Baron RH, Fey JV, Borgen PI, Stempel MM, Hardick KR, Van Zee KJ (2007) Eighteen sensations after breast cancer surgery: a 5-year comparison of sentinel lymph node biopsy and axillary lymph node dissection. Ann Surg Oncol 14 (5): 1653–1661
Bennett MI, Smith BH, Torrance N, Potter J (2005) The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain 6 (3): 149–158
Bouhassira D, Attal N (2009) All in one: is it possible to assess all dimensions of any pain with a simple questionnaire? Pain 144 (1-2): 7–8
Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lantéri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E (2005) Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 114 (1-2): 29–36
Broadbent E, Petrie KJ, Alley PG, Booth RJ (2003) Psychological stress impairs early wound repair following surgery. Psychosom Med 65 (5): 865–869
Bruce J, Drury N, Poobalan AS, Jeffrey RR, Smith WC, Chambers WA (2003) The prevalence of chronic chest and leg pain following cardiac surgery: a historical cohort study. Pain 104 (1-2): 265–273
Bruce J, Poobalan AS, Smith WC, Chambers WA (2004) Quantitative assessment of chronic postsurgical pain using the McGill Pain Questionnaire. Clin J Pain 20 (2): 70–75
Cancer Research UK (2011) Statistics for breast cancer. In Cancer Help UK Vol. 2012. CRUK: London
Cleeland CS, Ryan KM (1994) Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med 23 (2): 129–138
Fecho K, Miller NR, Merritt SA, Klauber-Demore N, Hultman CS, Blau WS (2009) Acute and persistent postoperative pain after breast surgery. Pain Med 10 (4): 708–715
Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, Kissin M, Mansel RE (2006) Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat 95 (3): 279–293
Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR (2011) Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol 29 (9): 1101–1109
Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H (2009) Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 302 (18): 1985–1992
Gerbershagen HJ, Dagtekin O, Gaertner J, Petzke F, Heidenreich A, Sabatowski R, Ozgür E (2010) Preoperative chronic pain in radical prostatectomy patients: preliminary evidence for enhanced susceptibility to surgically induced pain. Eur J Anaesthesiol 27 (5): 448–454
Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thürlimann B, Senn HJ (2003) Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol 21 (17): 3357–3365
Gottschalk A, Ochroch EA (2008) Clinical and demographic characteristics of patients with chronic pain after major thoracotomy. Clin J Pain 24 (8): 708–716
Haanpää M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, Cruccu G, Hansson P, Haythornthwaite JA, Iannetti GD, Jensen TS, Kauppila T, Nurmikko TJ, Rice AS, Rowbotham M, Serra J, Sommer C, Smith BH, Treede RD (2011) NeuPSIG guidelines on neuropathic pain assessment. Pain 152 (1): 14–27
Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M (2010) It's not over when it's over: long-term symptoms in cancer survivors – a systematic review. Int J Psychiatry Med 40 (2): 163–181
IASP (1994) (International Association for the Study of Pain) Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Pain 24 (S): 226
Ip HY, Abrishami A, Peng PW, Wong J, Chung F (2009) Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology 111 (3): 657–677
Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice AS, Treede RD (2011) A new definition of neuropathic pain. Pain 152 (10): 2204–2205
Johnston M (1986) Pre-operative emotional states and post-operative recovery. Adv Psychosom Med 15: 1–22
Johnston M, Vögele C (1993) Benefits of psychological preparation for surgery: a meta-analysis. Ann Behav Med 15: 12
Katz J (2011) One man's risk factor is another man's outcome: Difference in risk factor profiles for chronic postsurgical pain maintenance vs transition. Pain 153 (3): 505–506
Katz J, Poleshuck EL, Andrus CH, Hogan LA, Jung BF, Kulick DI, Dworkin RH (2005) Risk factors for acute pain and its persistence following breast cancer surgery. Pain 119 (1-3): 16–25
Kehlet H, Dahl JB (2011) Assessment of postoperative pain – need for action!. Pain 152 (8): 1699–1700
Kehlet H, Jensen TS, Woolf CJ (2006) Persistent postsurgical pain: risk factors and prevention. Lancet 367 (9522): 1618–1625
Kehlet H, Rathmell JP (2010) Persistent postsurgical pain: the path forward through better design of clinical studies. Anesthesiology 112 (3): 514–515
Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW (2007) The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med 30 (1): 77–94
Loukas M, Hullett J, Louis RG, Holdman S, Holdman D (2006) The gross anatomy of the extrathoracic course of the intercostobrachial nerve. Clin Anat 19 (2): 106–111
Macdonald L, Bruce J, Scott NW, Smith WC, Chambers WA (2005) Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer 92 (2): 225–230
Meevissen YM, Peters ML, Alberts HJ (2011) Become more optimistic by imagining a best possible self: effects of a two week intervention. J Behav Ther Exp Psychiatry 42 (3): 371–378
Pavlin DJ, Sullivan MJ, Freund PR, Roesen K (2005) Catastrophizing: a risk factor for postsurgical pain. Clin J Pain 21 (1): 83–90
Peters ML, Sommer M, de Rijke JM, Kessels F, Heineman E, Patijn J, Marcus MA, Vlaeyen JW, van Kleef M (2007) Somatic and psychologic predictors of long-term unfavorable outcome after surgical intervention. Ann Surg 245 (3): 487–494
Peters ML, Sommer M, van Kleef M, Marcus MA (2010) Predictors of physical and emotional recovery 6 and 12 months after surgery. Br J Surg 97 (10): 1518–1527
Peterson C, Semmel A, von Baeyer C, Abramson L, Metalsky GI, Seligman MEP (1982) The attributional style questionnaire. Cogn Ther Res 6: 287–299
Peuckmann V, Ekholm O, Rasmussen NK, Groenvold M, Christiansen P, Møller S, Eriksen J, Sjøgren P (2009) Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. Eur J Pain 13 (5): 478–485
Powell R, Bruce J, Johnston M, Vögele C, Scott N, Shehmar M, Roberts T (2010) Psychological preparation and postoperative outcomes for adults undergoing general anaesthesia (Protocol). Cochrane Database Syst Rev (8): Art. No. CD008646. doi:10.1002/14651858.CD008646
Powell R, Johnston M, Smith WC, King PM, Chambers WA, Krukowski Z, McKee L, Bruce J (2012) Psychological risk factors for chronic post-surgical pain after inguinal hernia surgery: a prospective cohort study. Eur J Pain 16 (4): 600–610
Rainville P, Bao QV, Chrétien P (2005) Pain-related emotions modulate experimental pain perception and autonomic responses. Pain 118 (3): 306–318
Rawal N, Allvin R (1998) Acute pain services in Europe: a 17-nation survey of 105 hospitals. The EuroPain Acute Pain Working Party. Eur J Anaesthesiol 15 (3): 354–363
Richards M, Corner J, Maher J (2011) The National Cancer Survivorship Initiative: new and emerging evidence on the ongoing needs of cancer survivors. Br J Cancer 105 (Suppl 1): S1–S4
Scheier MF, Carver CS (1987) Dispositional optimism and physical well-being: the influence of generalized outcome expectancies on health. J Pers 55 (2): 169–210
Scheier MF, Matthews KA, Owens JF, Magovern GJ, Lefebvre RC, Abbott RA, Carver CS (1989) Dispositional optimism and recovery from coronary artery bypass surgery: the beneficial effects on physical and psychological well-being. J Pers Soc Psychol 57 (6): 1024–1040
Shack L, Jordan C, Thomson CS, Mak V, Møller H Registries UAoC (2008) Variation in incidence of breast, lung and cervical cancer and malignant melanoma of skin by socioeconomic group in England. BMC Cancer 8: 271
Smith WC, Bourne D, Squair J, Phillips DO, Chambers WA (1999) A retrospective cohort study of post mastectomy pain syndrome. Pain 83 (1): 91–95
Spielberger C, Gorsuch R, Lushene P, Vagg P, Jacobs G (1983) Manual for the State-Trait Anxiety Inventory (Form Y). http://www.mindgarden.com/products/staisad.htm Mind Garden Inc
Srikandarajah S, Gilron I (2011) Systematic review of movement-evoked pain vs pain at rest in postsurgical clinical trials and meta-analyses: a fundamental distinction requiring standardized measurement. Pain 152 (8): 1734–1739
Steegers MA, Wolters B, Evers AW, Strobbe L, Wilder-Smith OH (2008) Effect of axillary lymph node dissection on prevalence and intensity of chronic and phantom pain after breast cancer surgery. J Pain 9 (9): 813–822
Tasmuth T, Estlanderb AM, Kalso E (1996) Effect of present pain and mood on the memory of past postoperative pain in women treated surgically for breast cancer. Pain 68 (2-3): 343–347
Watson D, Clark LA, Tellegen A (1988) Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54 (6): 1063–1070
Watson D, Pennebaker JW (1989) Health complaints, stress, and distress: exploring the central role of negative affectivity. Psychol Rev 96 (2): 234–254
Wilder-Smith OH, Tassonyi E, Arendt-Nielsen L (2002) Preoperative back pain is associated with diverse manifestations of central neuroplasticity. Pain 97 (3): 189–194
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67 (6): 361–370
Acknowledgements
The Recovery Study team comprises: Julie Bruce (Principal Investigator), Alison Thornton, Neil Scott (Co-Investigator, CI), Susan Marfizo, Marie Johnston (CI), Rachael Powell, Steven Heys (CI), Alastair Thompson (CI), Cairns Smith, Mary Wells, Alastair Chambers (CI), Peter Fayers and Ian Daltrey. We thank senior breast care practitioners who represent nursing teams from Grampian, Tayside and Highland Regions: Val Bain, Gillian Little, Ann Kemp, Karen Daltrey, Evelyn MacDonald. We also thank medical secretaries and surgeons for assisting with data collection and other staff who contributed to the project: Jeannette Davidson, Katie Wilde, Marcus Beasley, Dorothy Scott and Fiona MacAllan. Dr William Macrae and Dr Fiona Blyth commented on early versions of questionnaires. The study was funded by Cancer Research UK Project Grant C23143/A7531. Finally, our very grateful thanks to all the women who participated in the study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Bruce, J., Thornton, A., Scott, N. et al. Chronic preoperative pain and psychological robustness predict acute postoperative pain outcomes after surgery for breast cancer. Br J Cancer 107, 937–946 (2012). https://doi.org/10.1038/bjc.2012.341
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.341
Keywords
This article is cited by
-
Predictors of postoperative pain six months after breast surgery
Scientific Reports (2023)
-
Chronic pain and its correlates among long-term breast cancer survivors
Journal of Cancer Survivorship (2023)
-
Association between chronic pain and quality of life in long-term breast cancer survivors: a prospective analysis
Breast Cancer (2023)
-
Characteristics and distribution of chronic pain after mastectomy and breast reconstruction: a long-term prospective cohort study
Surgery Today (2023)
-
The association between emotion regulation and pain during the immediate postpartum period
Archives of Gynecology and Obstetrics (2023)