Abstract
Whether plasticity of white matter (WM) microstructure relates to therapeutic response in major depressive disorder (MDD) remains uncertain. We examined diffusion tensor imaging (DTI) correlates of WM structural connectivity in patients receiving electroconvulsive therapy (ECT), a rapidly acting treatment for severe MDD. Tract-Based Spatial Statistics (TBSS) applied to DTI data (61 directions, 2.5 mm3 voxel size) targeted voxel-level changes in fractional anisotropy (FA), and radial (RD), axial (AD) and mean diffusivity (MD) in major WM pathways in MDD patients (n=20, mean age: 41.15 years, 10.32 s.d.) scanned before ECT, after their second ECT and at transition to maintenance therapy. Comparisons made at baseline with demographically similar controls (n=28, mean age: 39.42 years, 12.20 s.d.) established effects of diagnosis. Controls were imaged twice to estimate scanning-related variance. Patients showed significant increases of FA in dorsal fronto-limbic circuits encompassing the anterior cingulum, forceps minor and left superior longitudinal fasciculus between baseline and transition to maintenance therapy (P<0.05, corrected). Decreases in RD and MD were observed in overlapping regions and the anterior thalamic radiation (P<0.05, corrected). Changes in DTI metrics associated with therapeutic response in tracts showing significant ECT effects differed between patients and controls. All measures remained stable across time in controls. Altered WM microstructure in pathways connecting frontal and limbic areas occur in MDD, are modulated by ECT and relate to therapeutic response. Increased FA together with decreased MD and RD, which trend towards normative values with treatment, suggest increased fiber integrity in dorsal fronto-limbic pathways involved in mood regulation.
Similar content being viewed by others
Introduction
Despite the availability of effective treatments for major depressive disorder (MDD), only half to two-thirds of patients can expect to achieve remission.1, 2, 3, 4 Understanding the mechanisms of treatment response is thus a priority for improving health outcomes. Available evidence supports that brain networks involved in mood, vegetative states and cognition contribute to MDD. Neuroimaging research points to structural and functional abnormalities in components of these networks, which include the anterior cingulate (ACC), dorsolateral (DLPFC), dorsomedial (DMPFC) and ventral prefrontal cortex (VPFC) and hippocampal, amygdalar, thalamic and striatal subcortical centers.5, 6, 7 Altered connectivity between network components is also shown to associate with MDD.7, 8, 9 As white matter (WM) architecture accounts for structural connectivity, and the strength and persistence of functional connectivity is shown to reflect and be constrained by structural connectivity,10,11 microstructural properties of WM may have an important role in the pathophysiology of MDD and in treatment response.
A growing number of cross-sectional studies have used diffusion tensor imaging (DTI) to investigate WM microstructure in MDD. Despite different methodological approaches, most investigations report MDD-related reductions of fractional anisotropy (FA), used as an index of WM integrity. For example, a systematic review, though focusing more broadly on affective disorders, reported smaller FA in patients relative to controls in 21 of 27 studies, where abnormalities in frontal and temporal regions were most reproducible.12 A meta-analysis including seven studies of MDD showed smaller FA in the superior longitudinal fasciculus (SLF),13 an association pathway connecting frontal, temporal and parietal regions.14 A more recent meta-analysis including 11 DTI studies (four overlapping with the prior meta-analysis) pointed to smaller FA in frontal lobe WM, as well as in right fusiform and occipital lobe WM in MDD.9 Altered FA in the sagittal striatum,15 which includes intersecting fibers from the SLF, and the inferior longitudinal (ILF) and fronto-occipital fasciculi (IFO),16, 17, 18, 19, 20 the internal and external capsule,16,19,21,22 cingulate/cingulum15,21,23, 24, 25 and other frontal association pathways18,24, 25, 26 are further reported in independent studies of MDD. Thus, though frontal, limbic and striatal connections are implicated, the regional pattern of altered WM structural connectivity in MDD requires clarification. Targeting changes in WM microstructure in association with treatment may help determine which WM pathways are most relevant to disease processes as well as indicate how neuroplasticity in structural networks relates to therapeutic response.
Previous reports show that WM abnormalities relate to illness severity and are pronounced in treatment-resistant depression.17,27, 28, 29, 30 However, longitudinal studies delineating the correlates and predictors of treatment response are few. One group using a whole brain voxel-based analysis approach not restricted to WM, showed treatment-related increases of FA in the left medial superior frontal cortex and decreased FA in the angular gyrus in 21 MDD patients after four weeks of guided image psychotherapy.31 Another group employing similar methods showed significant increases in FA in clusters in the left frontal gyrus in 17 MDD patients undergoing a 4-week trial of repetitive transcranial magnetic stimulation, but not in patients receiving sham stimulation.32 Electroconvulsive therapy (ECT), another brain stimulation technique used to treat treatment-resistant depression that elicits a more rapid and definitive response,33 provides a valuable model to examine treatment effects over relatively short intervals. One early study in patients with late-life MDD (n=8)34 showed increased FA post-ECT in WM sampled discretely from specific regions of interest (ROIs) in the frontal and temporal lobes.
To better determine the neurobiological effects of rapid treatment response, we used DTI to assess changes in WM microstructure in 20 patients with MDD followed prospectively while receiving ECT. Comparing patients with 28 controls at baseline established effects of diagnosis. Analyses employed FSL’s TBSS (Tract Based Spatial Statistics),35 allowing for the investigation of implicated tracts as well as for the unbiased exploration of other WM changes at the voxel level. Variations in FA as well as in radial (RD), axial (AD) and mean diffusivity (MD), which may point to particular properties of fiber integrity and coherence, were included as dependent measures. Associations with clinical response were examined in tracts showing significant treatment effects. Since neural models of MDD support a dysregulation between under reactive dorsal (DLPFC and DMPFC and dorsal ACC) and over active ventral (orbitofrontal, subgenual ACC, amygdala) fronto-limbic circuitry,36, 37, 38, 39 we hypothesized that treatment-related changes in structural connectivity would occur in dorsal and ventral pathways involved in mood regulation and emotion.
Materials and Methods
Participants
Patients were recruited from individuals independently scheduled to receive ECT as part of their routine care at the University of California, Los Angeles (UCLA) Resnick Neuropsychiatric Hospital. Eligibility required patients to have recurrent MDD and a DSM-IV TR diagnosis of a major depressive episode as confirmed by psychiatric evaluation and the Mini-International Neuropsychiatric Interview (M.I.N.I.).40 Patients with comorbid psychiatric disorders or dementia were excluded. Additional exclusionary criteria included first episode depression, onset after 50 years, depression related to a serious medical illness, and ECT or other neuromodulation treatments (vagal nerve stimulation, repetitive transcranial magnetic stimulation) within 6 months of current index ECT series. All patients were tapered off medications including antidepressants, benzodiazepines and anticonvulsants for at least 48 to 72 h before enrolling in this study and receiving treatment.
ECT was administered using the ST (seizure threshold) titration method; individual STs were determined using established stimulus dosing techniques. Sixteen patients received right unilateral d’Elia lead placement and four patients received right unilateral as well as bilateral bitemporal lead placement during their index series. After seizure threshold was determined at the first ECT session, subsequent treatments were delivered at energy settings 5x ST for right unilateral ECT and at 1.5x ST for bilateral ECT.
Demographically similar control subjects were recruited from the Los Angeles area using flyers. Controls received M.I.N.I. screening40 and were excluded if they had a history of depression or any other psychiatric illness, and/or history of antidepressant use. Exclusion criteria for all subjects included history of alcohol or substance abuse within the past 6 months and/or dependence within the past 12 months, any neurological disorder, and any condition that would contraindicate MRI scanning.
Patients with MDD (n=20) received scanning and mood evaluations at three separate time points: (1) within 24 h before their first ECT session (baseline), (2) after the second ECT session occurring ~48 h after their first ECT session (time point 2) and (3) within 1 week of completion of their ECT index series (time point 3), at which time they transitioned to a maintenance therapy (range of ECT index: 9–19 ECT sessions as individually determined). Mood scales collected at each time point included the Hamilton Rating Scale for Depression (HAM-D, 17-item),41 the Montgomery-Åsberg Depression Rating Scale (MADRS)42 and the Quick Inventory of Depressive Symptomology Self-Report (QIDS-SR,16-item).43
To estimate the variance associated with serial scanning and to establish normative values, control subjects (n=28) were scanned twice at intervals similar to patient baseline and transition scans. Demographic and clinical information for all subjects is provided in Table 1. All participants provided written informed consent and the study was approved by the UCLA Institutional Review Board.
Image acquisition and processing
DTI data were acquired on a Siemens 3T Allegra MRI system with an RF transmit/receive head coil using a spin-echo, echo-planar sequence including 61 non-collinear, icosahedrally distributed directions, 10 b0 images and 55 axial slices (TR/TE: 7300/95 ms, b=0, 1000 s/ mm2; FOV: 24 cm, 96 × 96 matrix, 2.5 mm isotropic voxel size). For processing, images were corrected for slice prescription and for residual eddy current distortions and motion artifacts using combined nonlinear 2-dimensional and 3-dimensional rigid body registrations.45,46 The diffusion tensor was computed at each voxel using a linear least squares method.47 The resultant eigen values were used to compute FA (degree of anisotropic diffusion), AD (λ1, diffusivity along the principal axis), RD ((λ2+λ3)/2), diffusivity along the two minor axes) and MD (overall diffusivity). Preprocessing was performed using in-house software written in C.48,49 FA, AD, RD and MD images were scalp-edited using masks generated from the co-registered b0 images.50
Voxel-wise statistical analysis used validated TBSS workflows,35 which form part of FSL.50 In brief, FA images were first aligned within and across subjects and then to standard MNI152 space using combined nonlinear and affine registrations. A mean FA image (FA threshold: 0.2) was subsequently created and thinned to represent a FA skeleton common to all subjects and time points. The aligned FA data from each subject or time point was then projected onto this skeleton to allow voxel-wise comparisons within major WM pathways. The transformation files and skeleton projection vectors generated for the FA images were applied to the MD, RD and AD images to allow comparison of these diffusion metrics in the same common space.
Statistical analysis
FSL’s Randomise tool (http://www.fmrib.ox.ac.uk/fsl/randomise/index.html), which combines the general linear model (GLM) with permutation testing, was used for voxel-based analysis of each diffusion metric. Paired t-tests established longitudinal changes in patients scanned at three time points, with each of the time points compared pairwise, and in controls scanned at two time points. Cross-sectional comparisons between patients at baseline and controls (using the first time point only) controlled for age and gender. FSL’s threshold-free cluster enhancement (TFCE) methods corrected for multiple statistical testing using 5000 randomly generated permutations and a threshold t>2, P<0.05. The anatomical locations of clusters showing significant effects were identified using the Johns Hopkins University (JHU) DTI-based WM atlas.51
In follow-up analyses, FA, AD, RD or MD values were also averaged, plotted and compared within WM ROIs showing significant longitudinal effects of ECT. The same ROIs were used to compare differences between patients and controls and to examine associations with clinical response. Associations with therapeutic response were assessed using the general linear mixed model (GLMM) including HAM-D, MADRS or QIDS ratings as covariates of interest, time point as a repeated measure and subject as a random factor. Finally, to determine whether baseline measures may predict subsequent clinical response, correlations between baseline DTI measures and change in clinical scores between baseline and time point 3 were examined.
Results
Subject characteristics
Patient and control groups did not differ significantly in age F(1,47)=0.13, P=0.71, gender X2(1,47)=0.96, P=0.65 or education F(1,47)=1.89, P=0.17.
Longitudinal effects
HAM-D, MADRS and QIDS scores improved between baseline and time point 2, t(1,19)=4.33, 5.25 and 5.75, respectively, all P<0.001; time point 2 and 3, t(1,19)=3.90, 4.78 and 5.42, all P<0.001; and baseline and time point 3, t(1,19)=6.30; 6.91 and 7.92, all P<0.0001.
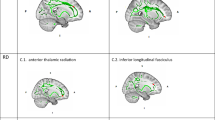
Patients showed significant increases in FA between baseline and time point 3 in the bilateral anterior cingulum, forceps minor, and the left SLF (P<0.05, corrected; Figure 1, top left). Though comparisons with time point 2 remained below the threshold of significance in voxel-level analysis, when averaged in tract ROIs showing significant overall effects of ECT (baseline versus the end of the ECT index), FA was shown as significantly increased between the second and third time points, t(1,19)=2.81, P<0.01 (Figure 1, top right).
Significant longitudinal effects of ECT and cross-sectional effects of diagnosis from voxel-based and ROI analysis. Left: significant increases in FA (top) shown in red, and decreases in MD (middle) and RD (bottom) shown in blue from voxel-based analyses comparing baseline and the end of the ECT index series (time point 3; P<0.05, corrected). Significant effects are superimposed onto the MNI 152 atlas, with the FA white matter skeleton for all subjects shown in green. Right: mean FA (top), mean MD (middle) and mean RD (bottom) averaged within regions showing significant ECT effects plotted by group and time point. The bold asterisks indicate significance between baseline and time point 3 corresponding to the TBSS analysis for each DTI metric shown on the right. Smaller asterisks indicate significance between groups or time point for tract-specific ROIs compared in follow-up analysis. DTI, diffusion tensor imaging; ECT, electroconvulsive therapy; FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity; ROI, region of interest.
Patients showed decreases in MD in the left anterior cingulum, left forceps minor and left anterior thalamic radiation (ATR) between baseline and time point 3 (P<0.05, corrected; Figure 1, middle left). MD averaged within ROIs showing significant overall effects of ECT, were again shown to vary between the second and third time points, t(1,19)=−2.51, P=0.02 (Figure 1, middle right).
Decreases in RD were observed in the same regions showing FA effects (forceps minor, anterior cingulum, and left SLF) between baseline and time point 3 (P<0.05, corrected; Figure 1, bottom left). RD in ROIs showing significant overall effects of ECT also differed between the second and third time points, t(1,19)=2.62, P=0.017 (Figure 1, bottom right). Means for FA, MD and RD from ROIs showing significant ECT effects are provided in Table 1.
Longitudinal effects were absent for AD. Significant differences between baseline and follow-up scanning were absent for all DTI metrics in controls. In Supplementary Figures 1–3, longitudinal effects of ECT are mapped after separating each tract exhibiting significant effects.
Cross-sectional effects
Though differences between diagnostic groups at baseline were not detected after correction for multiple comparisons at the voxel level, follow-up analyses revealed appreciable diagnostic group effects in the same tract ROIs showing significant longitudinal changes of ECT. Specifically, patients showed significantly smaller FA values in the cingulum, forceps minor, and left SLF compared with controls, F(1,47)=5.00, P=0.03 (Figure 1, top right). Larger MD values (Figure 1, middle right) and RD values (Figure 1, bottom right) were observed in overlapping regions, F(1,47)=6.07, P=0.018 and F(1,47)=4.34, P<0.043, respectively. No differences between diagnostic groups were observed when DTI measures were compared between controls and patients assessed at the second or third time points, all P>0.14.
Associations with clinical scores
Treatment-related increases in FA associated with decreased MADRS, F(1,20.90)=12.41, P<0.002; HAM-D, F(1,20.98)=13.41, P<0.001 and QIDS, F(1,21.24)=16.85, P<0.0001, ratings (Figure 2, top). Decreases in MD associated with decreased MADRS, F(1,20.35)=18.93, P<0.0001; HAM-D, F(1,20.68)=22.09, P=0.0001 and QIDS, F(1,21.49)=24.81, P=0.0001, scores (Figure 2, middle). Likewise, decreases in RD associated with decreased MADRS, F(1,20.89)=12.42, P<0.002; HAM-D, F(1,21.01)=14.19, P=0.001 and QIDS, F(1,21.51)=17.63, P=0.0001, ratings (Figure 2, bottom). DTI measures at baseline, however, were not shown to significantly associate with the extent of change in mood scales between baseline and the end of ECT index (all P>0.05).
Relationships between change in clinical ratings and change in FA, MD and RD within regions showing significant ECT effects. The Y axis shows mean FA (top), mean MD (middle) and mean RD (bottom) values from tract-specific ROIs. MADRS, HAM-D and QIDS scores are shown on the X axis. Values obtained from each patient are mapped in a different color with a data point for each time point (baseline, after the second ECT session and at the end of the ECT index series). Subject-specific regression lines indicate relationships between FA, MD and RD and MADRS, HAM-D and QIDS ratings across time. These relationships were significant for all DTI metrics (all P<0.001). DTI, diffusion tensor imaging; ECT, electroconvulsive therapy; FA, fractional anisotropy; HAM-D, Hamilton Rating Scale for Depression; MADRS, Montgomery–Åsberg Depression Rating Scale; MD, mean diffusivity; QIDS, Quick Inventory of Depressive Symptomology; RD, radial diffusivity; ROI, region of interest.
Discussion
The present study sought to address whether longitudinal changes in WM microstructure, examined in major tracts throughout the brain, occur in association with ECT and treatment-related clinical response. Cross-sectional differences between diagnostic groups addressed the presence of disease effects. Several key findings emerged from this investigation. In patients followed longitudinally, increases in FA and decreases in RD were shown in the anterior cingulum, the forceps minor and the dorsal aspect of the left SLF between baseline and the end of the ECT index series. Decreases in MD occurred in overlapping regions and in the anterior thalamic radiation, though were more left lateralized. Longitudinal changes within these pathways, which maintain more dorsal fronto-limbic connections, were associated with improved clinical response. In cross-sectional analysis, FA, MD and RD, measured in the same tract-specific ROIs exhibiting overall effects of ECT, were shown to differ between patients and controls at baseline. All DTI metrics remained stable over time in controls, and were not shown to vary between diagnostic groups once patients had initiated ECT, suggesting that ECT acts to normalize MDD-related abnormalities in the structural connectivity of dorsal fronto-limbic pathways.
Though reductions of FA are typically reported in prior cross-sectional DTI investigations of MDD, results remain mixed at the regional level.9,12,13 As apparent from two meta-analytic studies,9,13 the examination of relatively small (median: n=21; range: 15–52) and clinically and demographically heterogeneous samples may contribute to discrepancies in results. The use of larger voxel sizes, fewer gradient directions, voxel-based analysis methods sensitive to registration confounds or approaches that are limited to assessing arbitrarily selected regions that may not focus on WM exclusively may also account for mixed findings. The current study attempted to minimize some of these prior limitations by using higher angular resolution data and analysis methods more robust to registration confounds.35 Though differences between diagnostic groups were not observed at the voxel-level after correction procedures, significant differences in FA, MD and RD were observed in the same anatomical regions showing ECT-related neuroplasticity. By focusing on pathways showing microstructural changes in association with treatment, our findings thus indicate that decreases of FA and increases of RD and MD in pathways linking frontal and limbic structures may represent a biological marker of MDD.
FA varies in relation to AD and RD. However, the separate examination of these scalar metrics may better determine the neurobiological determinates of altered WM microstructure. Prior work in animals and humans suggest that decreases in AD may reflect axonal damage52,53 and/or pruning.54 In contrast, increases in RD may reflect decreased myelination.53,55,56 Larger MD may indicate myelin breakdown, decreased cellular density or increased extracellular volumes.57,58 Observations of increased FA with decreased RD in the current study thus suggest increased fiber coherence and organization that may relate to processes of myelination or other properties of fiber membranes. Observations of treatment-related decreases in MD similarly suggest plasticity relating to myelination, but may also reflect decreases of inflammation and/or related changes in cell density or extracellular volume. Though limitations of the tensor model may lead to a misattribution of biophysical properties in regions with crossing fibers,59 this potential confound is less likely to impact the current findings given that effects were observed in clusters extending across several major WM tracts.
Though very few DTI studies have assessed treatment effects in MDD, our results are compatible with one prior study showing increased FA in preselected frontal regions pre-to-post ECT in a preliminary investigation of late-life depression.34 Our results further demonstrate ECT-related changes in the structural connectivity of selected fronto-limbic pathways.60 Findings from functional and electrophysiological imaging have shown hypometabolism, decreased blood flow or activity in the dorsal ACC and hyperactivity or increased blood flow in more ventral limbic structures in MDD.39,61, 62, 63 Several treatment studies have also shown that ACC activity relates to and may predict treatment response.64, 65, 66, 67 These prior observations support models of MDD indicating a dysregulation between under reactive dorsal (DLPFC and DMPFC and dorsal ACC) and overactive ventral (orbitofrontal, subgenual ACC, amygdala) fronto-limbic circuitry with mediation by the thalamus and striatum.36, 37, 38, 39 This pattern of altered functional circuitry can be interpreted to reflect a dissociation between dorsal regions with preferential involvement in emotional regulation from ventral regions with primary involvement in emotion generation.36
The ACC is linked to both emotional processing as well as to pain circuitry.68,69 With its connections to frontal and limbic system substructures, including the amygdala, hippocampus and insula, the ACC is involved in avoidance learning, affective pain, emotional recall and salience monitoring (for negative stimuli in particular) and emotional processing modulated by cognitive activity.37,68, 69, 70 The forceps minor, which contains axonal projections to the lateral and medial prefrontal cortex through the anterior callosum, also forms part of fronto-limbic circuitry and may therefore assist in regulating depressive symptoms.39,63 Treatment-related changes in WM microstructure observed in the dorsal anterior cingulum, forceps minor and superior SLF (situated above the ACC and connecting parietal cortex, DLPFC and DMPFC14), and frontally projecting thalamic fibers, suggest plasticity in neural circuits most compatible with the dorsal network. Thus, treatment-related plasticity may be more closely related to improvements in control over emotional states to regulate mood.63 However, both direct and indirect bi-directional anatomical connections exist between nodes of dorsal and ventral networks, emphasizing interactions and overlaps between these systems.36,37
Though structural connectivity does not completely equate with functional connectivity, the present findings are also compatible with recent studies addressing altered functional connectivity in MDD, which point to dysregulation within default mode, affective and/or cognitive control networks that include the ACC, DLPFC, VPFC, DMPFC, limbic substructures and thalamus.71, 72, 73 Although few studies have addressed links between resting state functional connectivity and antidepressant response, a recent investigation of nine patients with MDD, who remained on psychotropic medications, showed decreased activity in the left DLPFC and correlated activity in the right DLPFC, DMPFC, ACC and left parietal and somatosensory cortex following ECT treatment (mean number of ECT sessions: 8.3)74 and relationships with clinical response. Though our findings of structural connectivity using DTI imply that ECT associates with increased rather than decreased connectivity in dorsal fronto-limbic circuits, WM pathways showing significant treatment effects maintain both direct and indirect connections with the functional regions implicated in this prior study.74
Although prior cross-sectional studies remain mixed with regard to the lateralization of DTI findings, both functional and structural asymmetries are reported in MDD. As the DLPFC is involved in cognitive control, findings of leftward effects in DLPFC functional connectivity74 and our results showing leftward effects in dorsal fronto-limbic pathways, particularly for MD, may suggest ECT acts to remediate mood regulation via cognitive control mechanisms that are more left lateralized. These observations are also compatible with a body of evidence suggesting depression is linked with right lateralized hyper-activity and left-lateralized hypo-activity,75 where ECT may act to modulate this imbalance through interhemispheric connections of the forceps minor. However, it is also possible that findings may be influenced by ECT lead placement as stimulation was targeted to the nondominant hemisphere in the majority of patients to minimize potential cognitive side effects. Cell sizes for patients receiving both unilateral and bilateral lead placement during treatment, however, were not large enough to examine separately.
Although this is the first study to our knowledge to demonstrate ECT-related structural plasticity within dorsal fronto-limbic pathways, some study limitations exist. First, although longitudinal analyses benefited from more powerful within-subjects modeling, only ROI analysis revealed significant diagnostic group effects, which appear more subtle. Second, non-tensor models applied to diffusion data may better resolve differences in WM regions with crossing fibers. Tractography approaches, either deterministic or probabilistic, may also provide benefits for targeting regional changes in WM structural connectivity through a priori selected seed regions. Finally, as the risk of relapse for ECT remains similar to other forms of antidepressant treatment, investigations following patients for longer time periods are necessary to determine long-term predictors of treatment response. In summary, the current study demonstrates ECT-related plasticity in WM pathways connecting frontal and limbic regions involved in mood regulation where increased fiber integrity associates with improved therapeutic response.
References
Rush AJ, Trivedi M, Fava M . Depression, IV: STAR*D treatment trial for depression. Am J Psychiatry 2003; 160: 237.
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006; 163: 28–40.
McGrath PJ, Stewart JW, Fava M, Trivedi MH, Wisniewski SR, Nierenberg AA et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry 2006; 163: 1531–1541.
Nierenberg AA, Fava M, Trivedi MH, Wisniewski SR, Thase ME, McGrath PJ et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry 2006; 163: 1519–1530.
Lorenzetti V, Allen NB, Fornito A, Yucel M . Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord 2009; 117: 1–17.
Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K et al. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci Res 2004; 50: 1–11.
Drevets WC, Price JL, Furey ML . Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 2008; 213: 93–118.
Mayberg HS . Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 2003; 65: 193–207.
Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neuroscience 2013; 38: 49–56.
Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA 2009; 106: 2035–2040.
Greicius MD, Supekar K, Menon V, Dougherty RF . Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 2009; 19: 72–78.
Sexton CE, Mackay CE, Ebmeier KP . A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry 2009; 66: 814–823.
Murphy ML, Frodl T . Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol Mood Anxiety Disord 2011; 1: 3.
Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 2005; 15: 854–869.
Kieseppa T, Eerola M, Mantyla R, Neuvonen T, Poutanen VP, Luoma K et al. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affective Disorders 2010; 120: 240–244.
Korgaonkar MS, Grieve SM, Koslow SH, Gabrieli JD, Gordon E, Williams LM . Loss of white matter integrity in major depressive disorder: evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum Brain Mapp 2011; 32: 2161–2171.
Cole J, Chaddock CA, Farmer AE, Aitchison KJ, Simmons A, McGuffin P et al. White matter abnormalities and illness severity in major depressive disorder. Br J Psychiatry 2012; 201: 33–39.
Wu F, Tang Y, Xu K, Kong L, Sun W, Wang F et al. Whiter matter abnormalities in medication-naive subjects with a single short-duration episode of major depressive disorder. Psychiatry Res 2011; 191: 80–83.
Zou K, Huang X, Li T, Gong Q, Li Z, Ou-yang L et al. Alterations of white matter integrity in adults with major depressive disorder: a magnetic resonance imaging study. J Psychiatry Neurosci 2008; 33: 525–530.
Versace A, Almeida JR, Quevedo K, Thompson WK, Terwilliger RA, Hassel S et al. Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol Psychiatry 2010; 68: 560–567.
Zhu X, Wang X, Xiao J, Zhong M, Liao J, Yao S . Altered white matter integrity in first-episode, treatment-naive young adults with major depressive disorder: a tract-based spatial statistics study. Brain Res 2011; 1369: 223–229.
Guo WB, Liu F, Chen JD, Xu XJ, Wu RR, Ma CQ et al. Altered white matter integrity of forebrain in treatment-resistant depression: a diffusion tensor imaging study with tract-based spatial statistics. Prog Neuropsychopharmacol Biol Psychiatry 2012; 38: 201–206.
Bae JN, MacFall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD . Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry 2006; 60: 1356–1363.
Blood AJ, Iosifescu DV, Makris N, Perlis RH, Kennedy DN, Dougherty DD et al. Microstructural abnormalities in subcortical reward circuitry of subjects with major depressive disorder. PLoS One 2010; 5: e13945.
Ouyang X, Tao HJ, Liu HH, Deng QJ, Sun ZH, Xu L et al. White matter integrity deficit in treatment-naive adult patients with major depressive disorder. East Asian Arch Psychiatry 2011; 21: 5–9.
Peng HJ, Zheng HR, Ning YP, Zhang Y, Shan BC, Zhang L et al. Abnormalities of cortical-limbic-cerebellar white matter networks may contribute to treatment-resistant depression: a diffusion tensor imaging study. BMC Psychiatry 2013; 13: 72.
Hoogenboom WS, Perlis RH, Smoller JW, Zeng-Treitler Q, Gainer VS, Murphy SN et al. Limbic system white matter microstructure and long-term treatment outcome in major depressive disorder: a diffusion tensor imaging study using legacy data. World J Biol Psychiatry 2012; 15: 122–134.
de Diego-Adelino J, Pires P, Gomez-Anson B, Serra-Blasco M, Vives-Gilabert Y, Puigdemont D et al. Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol Med 2014; 44: 1171–1182.
Taylor WD, Kuchibhatla M, Payne ME, Macfall JR, Sheline YI, Krishnan KR et al. Frontal white matter anisotropy and antidepressant remission in late-life depression. PLoS One 2008; 3: e3267.
Zhou Y, Qin LD, Chen J, Qian LJ, Tao J, Fang YR et al. Brain microstructural abnormalities revealed by diffusion tensor images in patients with treatment-resistant depression compared with major depressive disorder before treatment. Eur J Radiol 2011; 80: 450–454.
Wang T, Huang X, Huang P, Li D, Lv F, Zhang Y et al. Early-stage psychotherapy produces elevated frontal white matter integrity in adult major depressive disorder. PLoS One 2013; 8: e63081.
Peng H, Zheng H, Li L, Liu J, Zhang Y, Shan B et al. High-frequency rTMS treatment increases white matter FA in the left middle frontal gyrus in young patients with treatment-resistant depression. J Affect Disord 2012; 136: 249–257.
Kho KH, van Vreeswijk MF, Simpson S, Zwinderman AH . A meta-analysis of electroconvulsive therapy efficacy in depression. J ECT 2003; 19: 139–147.
Nobuhara K, Okugawa G, Minami T, Takase K, Yoshida T, Yagyu T et al. Effects of electroconvulsive therapy on frontal white matter in late-life depression: a diffusion tensor imaging study. Neuropsychobiology 2004; 50: 48–53.
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31: 1487–1505.
Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV . Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem 2011; 96: 553–563.
Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH . Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry 2012; 169: 693–703.
Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME . Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry 2007; 61: 198–209.
Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ . Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neuroscience 2007; 27: 8877–8884.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59 ((Suppl 20)): 22–33.
Hamilton M . A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62.
Williams JB, Kobak KA . Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry 2008; 192: 52–58.
Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003; 54: 573–583.
Oldfield RC . The Assessment and Analysis of Handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9: 97–113.
Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC . Automated image registration: I. General methods and intrasubject, intramodality validation. J Comp AssisTomogr 1998; 22: 139–152.
Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC . Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comp AssisTomogr 1998; 22: 153–165.
Basser PJ, Mattiello J, LeBihan D . Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 1994; 103: 247–254.
Clark K, Narr KL, O’Neill J, Levitt J, Siddarth P, Phillips O et al. White matter integrity, language, and childhood onset schizophrenia. Schizophr Res 2012; 138: 150–156.
Anderson E, Bai Z, Bischof C, Blackford S, Demmel J, Dongarra J . LAPACK Users' Guide. 3rd edn. Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1999.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23: S208–S219.
Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S . Fiber tract-based atlas of human white matter anatomy. Radiology 2004; 230: 77–87.
Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH . Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003; 20: 1714–1722.
Concha L, Gross DW, Wheatley BM, Beaulieu C . Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. NeuroImage 2006; 32: 1090–1099.
Bockhorst KH, Narayana PA, Liu R, Ahobila-Vijjula P, Ramu J, Kamel M et al. Early postnatal development of rat brain: in vivo diffusion tensor imaging. J Neurosci Res 2008; 86: 1520–1528.
Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH . Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002; 17: 1429–1436.
Ou X, Sun SW, Liang HF, Song SK, Gochberg DF . The MT pool size ratio and the DTI radial diffusivity may reflect the myelination in shiverer and control mice. NMR Biomed 2009; 22: 480–487.
Tievsky AL, Ptak T, Farkas J . Investigation of apparent diffusion coefficient and diffusion tensor anisotrophy in acute and chronic multiple sclerosis lesions. AJNR Am J Neuroradiol 1999; 20: 1491–1499.
Alexander AL, Lee JE, Lazar M, Field AS . Diffusion tensor imaging of the brain. Neurotherapeutics 2007; 4: 316–329.
Wheeler-Kingshott CA, Cercignani M . About ‘axial’ and ‘radial’ diffusivities. Magn Reson Med 2009; 61: 1255–1260.
Catani M, Howard RJ, Pajevic S, Jones DK . Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 2002; 17: 77–94.
Ebert D, Ebmeier KP . The role of the cingulate gyrus in depression: from functional anatomy to neurochemistry. Biol Psychiatry 1996; 39: 1044–1050.
Drevets WC, Savitz J, Trimble M . The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 2008; 13: 663–681.
Phillips ML . Understanding the neurobiology of emotion perception: implications for psychiatry. Br J Psychiatry 2003; 182: 190–192.
Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry 2001; 158: 405–415.
Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997; 8: 1057–1061.
Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Wiegand M et al. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry 1999; 156: 1149–1158.
Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 2001; 158: 899–905.
Vogt BA . Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005; 6: 533–544.
Phan KL, Wager T, Taylor SF, Liberzon I . Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 2002; 16: 331–348.
Meerwijk EL, Ford JM, Weiss SJ . Brain regions associated with psychological pain: implications for a neural network and its relationship to physical pain. Brain Imaging Behav 2013; 7: 1–14.
Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 2007; 62: 429–437.
Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry 2005; 57: 1079–1088.
Sheline YI, Price JL, Yan Z, Mintun MA . Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA 2010; 107: 11020–11025.
Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC . Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci USA 2012; 109: 5464–5468.
Hecht D . Depression and the hyperactive right-hemisphere. Neurosci Res 2010; 68: 77–87.
Acknowledgements
This study was supported by Award Number R01MH092301 from the National Institute of Mental Health and Award Number R00HD065832 (for KC) from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Lyden, H., Espinoza, R., Pirnia, T. et al. Electroconvulsive therapy mediates neuroplasticity of white matter microstructure in major depression. Transl Psychiatry 4, e380 (2014). https://doi.org/10.1038/tp.2014.21
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2014.21
This article is cited by
-
How electroconvulsive therapy works in the treatment of depression: is it the seizure, the electricity, or both?
Neuropsychopharmacology (2024)
-
Brain connectivity in major depressive disorder: a precision component of treatment modalities?
Translational Psychiatry (2023)
-
Longitudinal trajectory of response to electroconvulsive therapy associated with transient immune response & white matter alteration post-stimulation
Translational Psychiatry (2022)
-
Noninvasive neuromodulation of the prefrontal cortex in mental health disorders
Neuropsychopharmacology (2022)
-
White matter changes following electroconvulsive therapy for depression: a multicenter ComBat harmonization approach
Translational Psychiatry (2022)