Abstract
This work aimed to establish an effective approach to evaluate the quality of frozen fish, focusing on changes in fish tissue structure and chemical composition during storage. Fresh tilapia samples were treated by coating with tangerine peel (TP) extract and then stored at −4, −8 and −18 °C, respectively, for 40 days. The frozen fish tissues were analyzed for structural and chemical changes. Fractal dimension, which quantifies the porous structure formed in the tissue samples, texture properties including hardness and springiness, and moisture content and water activity all decreased during the storage, while the extents of lipid oxidation, measured as peroxide value and thiobarbituric acid concentration, and protein degradation, monitored with total volatile basic nitrogen and trichloroacetic acid soluble peptides, increased. The change rates of these parameters decreased with decreasing the storage temperature and by applying TP extract. A model was developed for predicting fractal dimension, which indicated the quality of preserved tilapia and thus can be used to predict the shelf life under different storage temperatures. The results demonstrated that TP extract could extend the shelf life of frozen tilapia by 35–45% by inhibiting changes in tissue structure, moisture loss, lipid oxidation and protein degradation during frozen storage.
Similar content being viewed by others
Introduction
Nile Tilapia (Oreochromis Niloticus) is an extensively farmed fish. Because of its excellent biological characteristics and edible value, it becomes a popular food material around the world1. However, like other aquatic products, tilapia is highly perishable2. Generally, the most common and effective method to maintain its freshness is frozen storage3. Low temperatures can delay the deterioration of fish, but it also inevitably brings negative impacts on fish tissue4. During frozen storage, composition changes and moisture loss result in a honeycomb microstructure in the fish tissue. Such porous structure is inherently irregular and difficult to describe5.
The fractal dimension is a geometry parameter characterized as the space-filling capacity of a space structure at a certain scale5, which can be used to describe the porous structure in some food materials including frozen tilapia6. Fractal dimension analysis was also used as a quantitative indicator reflecting the surface roughness of frozen tofu7. In addition, Kerdpiboona et al. established a fractal dimension model for describing the relationship between microstructural and physical properties of dried foods8.
In this study, the preservation effects of tangerine peel (TP, Citri reticulatae pericarpium)9 on the tissue structure and qualities of frozen tilapia were evaluated using fractal dimension along with measurements of changes in hardness and springiness, as well as chemical compositions due to moisture loss, lipid oxidation and protein decomposition. The results demonstrated the applicability and accuracy of fractal dimension analysis for predicting qualities and shelf life of frozen tilapia.
Materials and Methods
All methods in this study were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by South China University of Technology.
Preparation of TP extract
Fresh TP (obtained from Huping Mountain, Hunan Province, China) was dried in a convection oven at 50 °C for 48 h and hydro-distilled in a Clevenger-type apparatus (Kesijia Ltd., Beijing, China) for 6 h to obtain the extract10. The resulting extract was dried with hydrous sodium sulfate, filtered, and stored at 4 °C in brown sealed glass vials.
Sample preparation and preservation
Tilapia, with average weight of 447 ± 63 g, was obtained from Lianfeng Aquaculture Base of Guangzhou, Guangdong Province, China. The fish was kept in plastic tanks at 25 °C for 24 h. Then it was killed, skinned, processed into butterfly-like fillets and thoroughly washed in dilute NaCl solutions. The resulting fillets were divided into 6 groups. Three of them were control groups without TP treatment; the other three groups were treated with TP extract by immersing the samples in an aqueous solution containing 0.2% v/v TP extract at ~15 °C for 30 min. These samples were frozen and stored in freezers (BCD-235NCQE, Le-jin Company, China) at −4 ± 0.2 °C, −8 ± 0.4 °C, and −18 ± 0.5 °C, respectively, for 40 days. Samples were taken every 10 days from the freezers for structural and chemical analyses. Each measurement was performed with at least three repeats in three different samples. The results were obtained by averaging. The data was processed using SPSS 17.0 for statistical analysis. One way analysis of variance was conducted to compare the effects under different storage times (p < 0.05). The least significance difference (LSD) test was used to determine differences at a = 0.0511.
Microscopic tissue imaging and fractal dimension analysis
The micro-sections of tilapia samples were prepared using the method described by He et al.6. The fillets were imbedded in frozen OCT (optimal cutting temperature) agents and cut into 20 μm slices using a freezing microtome (Model CM 1900; Leica Co. Ltd, USA). Then, the slices were stained with H&E method and images were obtained under an optical microscope (BX51; Olympus, USA). Fractal dimension values were obtained by the box-counting method12. The optical microscope image of a tissue sample was divided into square sub-boxes with a variable length denoted as ε. The total number of sub-boxes with more than 50% of its tissues was then counted as N(ε). The relationship between ε and N (ε) can be described by the following equation:

where d is the fractal dimension of the divided image and a is a constant. By plotting log N(ε) vs. log ε, the fractal dimension d can be obtained from the slope of the regression line (see Fig. S1 in Supplemental Materials).
Texture profile analysis
Texture profile analysis (TPA) was performed under the conditions described in a similar study13. A 6 mm cylindrical probe was used with 5 g trigger load and 5 mm/s test speed. Twice compressions with 5 mm depth were performed on the same point of each sample and separated by a 5 s interval. The TPA parameters including hardness and springiness were obtained from the measured force (N), area (Ns) and distance (mm) between peak heights.
Moisture content
The moisture content (MC) was estimated from the weights before and after drying to a constant weight in a 105 °C oven14. Water activity (Aw) was measured with minced tilapia tissue in a portable Aw instrument (Hygro Palm, Rotronic Company, Switzerland)15.
Lipid oxidation
Peroxide value (PV) was measured using the method reported by Lea16. Fifty grams of tilapia were minced and extracted with a mixture of 50 mL distilled water, 100 mL methanol, and 100 mL chloroform. After dissolving 1 g liquid extract in a mixture of 10 mL chloroform and 15 mL acetic acid, 1 mL saturated potassium iodide was added and the mixture was kept in the dark for 10 min. Then, 30 mL distilled water and 1 mL starch solution (1% w/v) were added, and the solution was titrated with 0.01 mol equiv/L Na2S2O3 until colorless. The PV was calculated from the dosage of Na2S2O3.
Thiobarbituric acid (TBA) was analyzed as described by Li et al. with some modifications17. One gram of tilapia was minced, dissolved in 5 mL of 4% v/v 1-butanol aqueous solution, pipetted into a dry stoppered test tube, mixed with 5 ml TBA reagent (200 mg 2-TBA dissolved in 100 mL 1-butanol), and incubated in a water bath at 95 °C for 120 min. After cooling, the absorbance at 530 nm of the mixture was measured to calculate the TBA.
Protein decomposition
Total volatile basic nitrogen (TVB-N) was measured using the semi-micro Kjeldahl method18. Ten grams of tilapia were minced, mixed with 50 mL distilled water, stirred for 30 min and then filtered. The filtrate was alkalinized with 10% suspension liquid of MgO and submitted to a Kjeldahl Apparatus (KDY-9820, Beijing, China). The volatile basic nitrogen or ammonia was absorbed in an acid receiver and determined by titration with 0.01 M HCl solution.
Trichloroacetic acid (TCA) soluble peptides were analyzed using the Lowry method19. Three grams of tilapia were minced and homogenized with 27 mL cold 5% (w/v) TCA. The homogenate was kept in ice for 30 min and centrifuged at 10000 rpm for 5 min at 4 °C. The soluble peptides in the supernatant were then determined and expressed as mmol tyrosine/g muscle.
Results and Discussion
Effects of frozen storage on tilapia tissue structure and texture
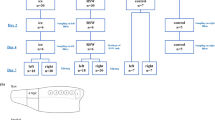
Changes in tilapia tissue structure and morphology during frozen storage at −18 °C (a common temperature for industrial storage), −8 °C (a temperature often used in consumers’ fridge), and −4 °C (a usual temperature for partially frozen storage)20 were studied. These three temperatures are representative in aquatic products storage21. Figure 1 shows the microscopic images of micro-sections of tilapia tissues after 20 days and 40 days at −4 °C, −8 °C and −18 °C. Compared to the initial samples, there were increasing void spaces (white) inside the frozon tissue (red) during storage22. Initially, tilapia tissues had only a few small void spaces, which grew into larger irregular pores over time23, which could be attributed to fluids migration caused by the ice crystallization and muscle fiber disarrangement caused by protein decomposition6. The increases in the void spaces were faster at higher temperatures. Interestingly, TP extract coating significantly hindered the structural change and was able to maintain tilapia tissue with fewer and smaller void spaces, especially at −18 °C.
Changes in tilapia tissue structure can be quantitatively analyzed by measuring the fractal dimension, which is sensitive enough to distinguish tiny differences in the structure, size, or area fraction24,25. Figure 2 shows the fractal dimension values of tilapia samples at various frozen temperatures and storage times. In general, fractal dimension decreased with increasing the temperature and storage time, and the decrease was significantly slower for samples treated with TP extract.
Changes in the tissue structure of tilapia should also affect its texture properties7. As shown in Fig. 3a,b, both hardness and springiness of frozen tilapia tissues also decreased over time, in similar trends to those for fractal dimension. Clearly, the increasingly porous structure, as quantified by fractal dimension, formed during frozen storage resulted in the declines in hardness and springiness. As expected, TP extract treatment slowed down the declines in these texture properties.
Changes in moisture content during frozen storage
Variations in tissue structure and texture of tilapia tissue are greatly affected by the moisture content and chemical compositions26. During frozen storage, the crystallized tissue fluids greatly destroyed the structure of the sample tissue27, while the loss of moisture due to sublimation created the honeycomb microstructure with large void spaces in the muscle tissue28. Figure 3c,d shows changes in the moisture content (MC) and water activity in frozen tilapia tissues. After 40 days, MC in the control group reduced 25.3%, 22.3% and 16.4% at −4 °C, −8 °C and −18 °C, respectively, from the initial value of 80.18 ± 3.15%. In contrast, tilapia tissues treated with the TP extract lost only 19.0%, 17.2% and 12.0%, respectively. Similar decreasing trends in Aw were also observed (Fig. 4b). The classical Brunauer–Emmet–Teller model has been used to describe the close relationship between fractal dimension and moisture content29,30. Based on the fractal generalization of the Frenkel-Holsi-Hill (FHH) equation, Pfeifer et al. showed that the dimension of fractal surface dfs was governed by the slope of the linear plot of moisture content vs. ln h, where h was the relative pressure31. It is thus clear that moisture lost during frozen storage can be quantitatively monitored through changes in fractal dimension.
Changes in chemical composition
Protein and lipid are two major organic components in fish tissue32. A chemical composition analysis showed that fresh tilapia tissue contained 14.70 ± 0.63% protein and 2.94 ± 1.19% lipid. Changes in protein and lipid contents in frozen tilapia also occurred during storage, which would reduce the water-retaining capability of the tissue and result in faster moisture loss. It can also directly disarrange muscle fibers and change tissue structure and texture33,34.
Lipid oxidation
Changes in the lipid content usually can be quantified by monitoring the degree of lipid oxidation, which is a significant process of chemical deterioration in the refrigerated aquatic products35. In this process, free radicals “stolen” electrons from the polyunsaturated fatty acids which contain multiple double bonds in cell membranes. It impacts water-retaining capability of tissue and results in the variations in the tissue structure36,37. PV and TBA are two common parameters for determining the degree of lipid oxidation38. In general, both PV and TBA in frozen tilapia increased with the storage time (Fig. 4a,b). The tilapia tissue had the initial PV of ~0.76 meq/kg, which increased to 3.69, 2.35, and 1.48 meq/kg in the control samples after 40 days at −4 °C, −8 °C and −18 °C, respectively (Fig. 4a). In contrast, the TP extract treated tilapia tissues had significantly lower PV of 2.29, 1.34 and 1.06 meq/kg at the respective storage temperatures. Similar trends were also observed with TBA, which increased from the initial 0.58 mg MDA/kg to 2.29, 1.31, and 0.93 mg MDA/kg for the control and from 0.51 MDA/kg to 1.39, 0.88, and 0.74 mg MDA/kg for the treated samples after 40 days at −4 °C, −8 °C and −18 °C, respectively (Fig. 4b). Since lipid oxidation is mainly associated with unsaturated fats exposed to oxygen or air, TP extract as a protective barrier on the sample surface can slow down lipid oxidation by deterring the contact with oxygen.
Protein decomposition
TVB-N and TCA soluble peptides are parameters useful for monitoring protein decomposition31. Both TVB-N and TCA soluble peptides in frozen tilapia increased significantly during frozen storage (Fig. 4c,d). As expected, the increases were slower at lower temperatures and with TP extract treatment. The muscle fiber in tilapia tissue composed mainly proteins, and therefore protein decomposition would greatly affect tissue morphology and texture39. Accordingly, changes in TVB-N and TCA soluble peptides would also affect the fractal dimension.
Fractal dimension as the key parameter for shelf-life prediction
Pearson correlation coefficient, which is widely used to determine the degree of linear dependence between two variables6, was calculated to determine correlations between fractal dimension and various texture and chemical parameters during frozen storage of tilapia (see Fig. S2 in Supplemental Materials). Table 1 shows that all have a Pearson correlation coefficient of ±0.95. This result indicated that fractal dimension can be employed as a reliable quality index to reveal variations in chemical and texture parameters quantitatively.
The shelf-life of the tilapia samples was predicted by first-order kinetics model with using fractal dimension of tilapia tissues as the key quality factor40. The model can be expressed as Eq. 2.

where t is the storage time (d), d0 is the initial value of fractal dimension, dt is the fractal dimension value at storage time t, and kT is the rate constant at storage temperature T.
Additionally, the relationship between the rate constant kT and the storage temperature T can be shown by the Arrhenius equation as follows41:

where k0 is the pre-exponential factor (d−1), Ea is the activation energy (kJ/mol), and R is the gas constant (8.314 J/mol K). T, kT, k0, and Ea are constants associated with the physical nature of the reaction system.
Incorporating Eq. 2 with Eq. 3, a global equation can be formulated:

With using the data of fractal dimension in the Fig. 2, activation energy Ea of the control samples was calculated as −1.104 × 1013 kJ/mol, and pre-exponential factor k0was 9883.975 d−1. Meanwhile, Ea of treated samples was −2.343 × 1013 kJ/mol, and k0was 10179.648 d−1. Accordingly, the first-order kinetics equation of fractal dimension was obtained as follows:


In addition, the maximum of fractal dimension in spoiled tilapia samples were 1.801 ± 0.0256. So the results of the shelf-life were obtained as listed in Table 2. The data was harmonious with similar researches6,40,41, which indicated that fractal dimension employed as a quality index was reliable. Additionally, the TP extracts treatment prolonged the shelf-life of the frozen tilapia about 40%. The results can confirm and quantify the effects of TP extracts on freshness of preserved fish reported in a previous study9.
Effects of TP extract
The result of GC-MS analysis of TP extracts was listed in Table S1. Limonene (68.44% w/w) and γ-terpinene (18.39% w/w) made up 85% of total TP extract. Limonene has been proved as an effective agent to inhibit the activity of spoilage bacteria, and its molecule containing multiple double bonds, which can be an effective barrier against lipid oxidation as an alternative of polyunsaturated fatty acids to offer electronics42. γ-terpinene was also recorded as an important preservative agent because of its antibacterial and antioxidant effects to some extent2. Additionally, many other compounds was known to be able to play positive roles to maintain the chemical properties of preserved materials9,43,44,45,46. Therefore, as shown in Fig. 4, TP extract showed good preservation effect for frozen-stored tilapia with significant inhibition effects on of chemical degradation. The effects can accordingly reduce the variation in tissue structure and resulting fractal dimension of tilapia.
Conclusion
In this study, the frozen fish tissues with TP extracts treatment were analyzed for structural and chemical changes. During frozen storage, fractal dimension, which quantifies the porous structure formed in the tissue samples, as well as texture properties including hardness and springiness, and moisture content and water activity all decreased during the storage, while the extents of lipid oxidation, measured as peroxide value and thiobarbituric acid concentration, and protein degradation, monitored with total volatile basic nitrogen and trichloroacetic acid soluble peptides increased. The change rates of these parameters decreased with decreasing the storage temperature and by applying TP extract. With excellent correlations among each other, fractal dimension can reveal the variations of chemical parameters as well as texture parameters accurately. A shelf life prediction with using fractal dimension as the quality index demonstrated that TP extract could extend the shelf life of frozen tilapia by 35–45%.
Additional Information
How to cite this article: He, Q. et al. Quality Evaluation Focusing on Tissue Fractal Dimension and Chemical Changes for Frozen Tilapia with Treatment by Tangerine Peel Extracts. Sci. Rep. 7, 42202; doi: 10.1038/srep42202 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ho, S. C. & Kuo, C. T. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium) . Food Chem. Toxicol. 71, 176–182 (2014).
Yi, L. Z., Dong, N. P., Liu, S., Yi, Z. B. & Zhang, Y. Chemical features of Pericarpium Citri Reticulatae and Pericarpium Citri Reticulatae Viride revealed by GC–MS metabolomics analysis. Food Chem. 186, 192–199 (2015).
Mahto, R., Ghosh, S., Das, M. K. & Das, M. Effect of gamma irradiation and frozen storage on the quality of fresh water prawn (Macrobrachium rosenbergii) and tiger prawn (Penaeus monodon). LWT–Food Sci. Technol. 61, 573–582 (2015).
Baron, C. P., Hyldig, G. & Jacobsen, C. Does feed composition affect oxidation of rainbow trout (Oncorhynchus mykiss) during frozen storage? J. Agr. Food Chem. 57, 4185–4194 (2009).
Othmar, P. Fractals and analytical chemistry an old and new look. Fresenius J. Anal. Chem. 337, 172–175 (1990).
He, Q., Zhu, L., Shen, Y., Lin, X. D. & Xiao, K. J. Evaluation of the effects of frozen storage on the microstructure of tilapia (Perciformes: Cichlidae) through fractal dimension method. LWT-Food Sci. Technol. 64, 1283–1288 (2015).
Hagiwara, T., Wang, H. L., Suzuki, T. & Takai, R. Fractal analysis of ice crystals in frozen food. J. Agr. Food Chem. 50, 3085–3089 (2002).
Kerdpiboona, S., Kerrb, W. L. & Devahastina, S. Neural network prediction of physical property changes of dried carrot as a function of fractal dimension and moisture content. Food Research International 39, 1110–1118 (2006).
He, Q. & Xiao, K. J. The effects of tangerine peel (Citri reticulatae pericarpium) essential oils as glazing layer on freshness preservation of bream (Megalobrama amblycephala) during superchilling storage. Food Control 69, 339–345 (2016).
Qi, H., Wang, W. X., Dai, J. L. & Zhu, L. In vitro anthelmintic activity of Zanthoxylum simulansessential oil against Haemonchus contortus . Vet. Parasitol. 211, 223–227 (2015).
Ocaño-Higueraa, V. M. et al. Freshness assessment of ray fish stored in ice by biochemical, chemical and physical methods. Food Chem. 125, 49–54 (2011).
Liu, Y. et al. An improved differential box–counting method to estimate fractal dimensions of gray–level images. J. Vis. Commun. Image R. 25, 1102–1111 (2014).
Mohsin, M., Bakar, J. & Selamat, J. The effects on colour, texture and sensory attributes achieved by washing black tilapia flesh with a banana leaf ash solution. Int. J. Food Sci. Tech. 34, 359–363 (1999).
de Souza, M. L. R. et al. Kaftas prepared with V-shaped filleting chips of the Nile tilapia (Oreochromis niloticus) exposed to smoking techniques. Acta Sci-technol. 37, 287–291 (2015).
Tsironi, T. N. & Taoukis, P. S. Effect of processing parameters on water activity and shelf life of osmotically dehydrated fish filets. J. Food Eng. 123, 188–192 (2014).
Lea, C. H. Methods for determination peroxide in lipid. J. Sci. Food Agr. 3, 586–594 (1952).
Li, T. T. et al. Coating effects of tea polyphenol and rosemary extract combined with chitosan on the storage quality of large yellow croaker (Pseudosciaena crocea). Food Control 25, 101–106 (2012).
Cai, L. Y. et al. The effects of essential oil treatment on the biogenic amines inhibition and quality preservation of red drum (Sciaenops ocellatus) fillets. Food Control 56, 1–8 (2015).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Taoukisa, P. S., Koutsoumanisb, K. & Nychasb, G. J. E. Use of time–temperature integrators and predictive modelling for shelf life control of chilled fish under dynamic storage conditions. Int. J. Food Microbiol. 53, 21–31 (1999).
Rahman, M. S. The accuracy of prediction of the freezing point of meat from general models. J. Food Eng. 21, 127–136 (1994).
Chow, C. J., Wu, J. C., Lee, P. F. & Ochiai, Y. Effects of acid and alkaline pretreatment on the discoloration rates of dark muscle and myoglobin extract of skinned tilapia fillet during iced storage. Fisheries Sci. 75, 1481–1488 (2009).
Pons-Sanchez-Cascado, S., Vidal-Carou, M. C., Marine-Font, A. & Veciana-Nogues, M. T. Influence of the freshness grade of raw fish on the formation of volatile and biogenic amines during the manufacture and storage of vinegar-marinated anchovies. J. Agr. Food Chem. 53, 8586–8592 (2005).
Sarkar, N. & Chaudhuri, B. B. Multifractal and generalized dimensions of gray–tone digital images. Signal Process. 2, 181–190 (1995).
Sarkar, N. & Chaudhuri, B. B. An efficient approach to estimate fractal dimension of textural images. Pattern Recogn. 25, 1035–1041 (1992).
Cheng, J. H., Sun, D. W., Zeng, X. A. & Liu, D. Recent advances in methods and techniques for freshness quality determination and evaluation of fish and fish fillets: A Review. Crit. Rev. Food Sci. 55, 1012–1025 (2015).
Tejada, M. & Heras, C. M. Michael Kent Changes in the quality indices during ice storage of farmed Senegalese sole (Solea senegalensis). Eur Food Res Technol. 225, 225–232 (2007).
Stuchell, Y. M. & Krochta, J. M. Edible coatings on frozen King salmon: effect of whey protein isolate and acetylated monoglycerides on moisture loss and lipid oxidation. J. Food Sci. 60, 28–31 (1995).
Fripiat, J. J., Gatineau, L. & Van Damme, H. Multilayer physical adsorption on fractal surface. Langmuir 2, 562–567 (1986).
Adamson, A. W. Physical chemistry of surfaces (6th ed.). New York: John Wiley Sons. Morphometric traits as selection criteria for carcass yield and body (1997).
Pfeifer, P., Wu, Y. J., Cole, M. W. & Krim, J. Multilayer adsorption on a fractally rough surface. Phys. Rev. Lett. 62, 1997–2000 (1989).
Olafsdottir, G. et al. Methods to evaluate fish freshness in research and industry. Trends Food Sci. Tech. 8, 258–265 (1997).
Dhanapal, K. et al. Quality of ready to serve tilapia fish curry with pufa in retortable pouches. J. Food Sci. 75, 348–354 (2010).
Liu, X. C., Jiang, Y., Shen, S., Luo, Y. K. & Gao, L. Comparison of Arrhenius model and artificial neuronal network for the quality prediction of rainbow trout (Oncorhynchus mykiss) fillets during storage at different temperatures. LWT–Food Sci. Technol. 60, 142–147 (2015).
Medina, I., Gallardo, J. M., Gonzalez, M. J., Lois, S. & Hedges, N. Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. J. Agr. Food Chem. 55, 3889–3895 (2007).
Joaquin, H. J. F., Tolasa, S., Oliveira, A. C. M., Lee, C. M. & Lee, K. H. Effect of milk protein concentrate on lipid oxidation and formation of fishy volatiles in herring mince (Clupea harengus) during frozen storage. J. Agr. Food Chem. 56, 166–172 (2008).
Baron, C. P., Kjaersgard, I. V. H., Jessen, F. & Jacobsen, C. Protein and lipid oxidation during frozen storage of rainbow trout (Oncorhynchus mykiss). J. Agr. Food Chem. 55, 8118–8125 (2007).
Torres-Arreola, W., Soto-Valdez, H., Peralta, E., Cardenas-Lopez, J. L. & Ezquerra-Brauer, J. M. Effect of a low-density polyethylene film containing butylated hydroxytoluene on lipid oxidation and protein quality of sierra fish (Scomberomorus sierra) muscle during frozen storage. J. Agr. Food Chem. 55, 6140–6146 (2007).
Fan, W. J., Chi, Y. L. & Zhang, S. The use of a tea polyphenol dip to extend the shelf life of silver carp (Hypophthalmicthys molitrix) during storage in ice. Food Chem. 108, 148–153 (2008).
Yao, L., Luo, Y. K., Sun, Y. Y. & Shen, H. X. Establishment of kinetic models based on electrical conductivity and freshness indictors for the forecasting of crucian carp (Carassius carassius) freshness. J. Food Eng. 107, 147–151 (2011).
Ruhil, A. P., Singh, R. R. B., Jain, D. K., Patel, A. A. & Patil, G. R. A comparative study of kinetic and connectionist modeling for shelf–life prediction of Basundi mix . J. Food Sci. Technol. Mys. 48, 204–210 (2011).
Perigo, C. V. et al. The chemical composition and antibacterial activity of eleven Piper species from distinct rainforest areas in Southeastern Brazil. Ind. Crop. Prod. 94, 528–539 (2016)
Rambla, J. L. et al. Fruit volatile profiles of two citrus hybrids are dramatically different from those of their parents. J. Agr. Food Chem. 62, 11312–11322 (2014).
Ramezani, Z., Zarei, M. & Raminnejad, N. Comparing the effectiveness of chitosan and nanochitosan coatings on the quality of refrigerated silver carp fillets. Food Control 51, 43–48 (2015).
Gao, B., Chen, Y. L., Zhang, M. W., Xu, Y. J. & Pan, S. Y. Chemical composition, antioxidant and antimicrobial activity of Pericarpium Citri reticulatae essential oil. Molecules 16, 4082–4096 (2011).
Njoroge, S. M., Mungai, H. N., Koaze, H., Phi, N. T. L. & Sawamura, M. Volatile constituents of mandarin (Citrus reticulata blanco) peel oil from Burundi. J. Essent. Oil Res. 18, 659–662 (2006).
Acknowledgements
The authors are grateful for the financial support from Guangzhou Science and Technology Program Key Projects, China (201508020086).
Author information
Authors and Affiliations
Contributions
Qi He conducted the main work and wrote the first draft of the manuscript. Zhao Yang and Jingjing Wang performed some parts of the experimental work. Bin Gong did additional work reported in the revised manuscript. Kaijun Xiao and Shang-Tian Yang offered support and contributed to the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
He, Q., Yang, Z., Gong, B. et al. Quality Evaluation Focusing on Tissue Fractal Dimension and Chemical Changes for Frozen Tilapia with Treatment by Tangerine Peel Extract. Sci Rep 7, 42202 (2017). https://doi.org/10.1038/srep42202
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42202
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.