Abstract
Nuclear hyperpolarization is a phenomenon that can be used to improve the sensitivity of magnetic resonance molecular sensors. However, such sensors typically suffer from short hyperpolarization lifetime. Herein we report that [15N, D14]trimethylphenylammonium (TMPA) has a remarkably long spin–lattice relaxation time (1128 s, 14.1 T, 30 °C, D2O) on its 15N nuclei and achieves a long retention of the hyperpolarized state. [15N, D14]TMPA-based hyperpolarized sensor for carboxylesterase allowed the highly sensitive analysis of enzymatic reaction by 15N NMR for over 40 min in phophate-buffered saline (H2O, pH 7.4, 37 °C).
Similar content being viewed by others
Introduction
Hyperpolarization is a promising method for improving the sensitivity of magnetic resonance (MR) molecular sensors. During the polarization process, the nuclear spin state of the MR molecular sensor is polarized and the NMR sensitivity thereof is enhanced by a factor of 103–105 compared with that under thermally equilibrated conditions1.
The high sensitivity that can be achieved with hyperpolarized molecular sensors has led to the application of such systems for in vivo enzymatic analysis2,3,4,5. A representative example is hyperpolarized [1-13C]pyruvate, the use of which is being explored as a diagnostic tool for noninvasive analysis of tumor status3. In recent years, in addition to such biomedical applications, hyperpolarized molecules have been utilized in several research fields, including analysis of host–guest interactions6,7, monitoring of polymerization reactions8, and solid-surface characterization9. These examples show the increasing potential utility of NMR hyperpolarized techniques.
However, the hyperpolarized technique has a nonnegligible shortcoming; namely, a short lifetime of hyperpolarized state. The enhanced NMR signal decays rapidly under physiological conditions. This short lifetime has restricted the range of analysis subjects and has hampered the widespread application of this technique2.
To address this crucial issue, attention has focused on the development of molecular units that can retain the hyperpolarized state for longer periods10,11,12,13,14,15,16,17,18. The hyperpolarization lifetime is directly correlated with the spin–lattice relaxation time (T1) of nuclei. Under physiological conditions, T1 value of 13C nuclei in typical organic compounds used in hyperpolarized study is less than several tens of seconds; the T1 of widely used [1-13C]pyruvate is longer but is still in the range of 40–60 s.
Previously we developed molecular units [15N]trimethylphenylammonium ([15N]TMPA) (1) and [15N, D9]TMPA (2) (Fig. 1). The quaternary 15N atoms of 1 and 2 have a long T1 of 275 and 816 s (14.1 T, 30 °C, D2O), respectively18. By utilizing the unit as a platform, three types of hyperpolarized molecular sensors were designed.
Herein, we addressed the challenge of extending the hyperpolarization lifetime further. Based on the relaxation mechanism, we report an improved molecular unit, the T1 of which is 1128 ± 112 s (14.1 T, 30 °C, D2O). The long T1 allows that the hyperpolarized NMR signal can be detected for over 1 h.
Results and Discussion
To achieve a long T1 for the extended hyperpolarization lifetime, we considered a relaxation mechanism (equation 1)15,19,20,21,22. Generally, T1 is affected by dipole–dipole (DD) relaxation, spin–rotation (SR) relaxation, chemical shift anisotropy (SA) relaxation, scalar coupling (SC) relaxation, and the other relaxation terms including dipolar relaxation from dissolved oxygen. In small to medium size molecules, the DD interaction with neighboring 1H atoms is one of the main factors that shorten the T1 value.

The 15N atom in [15N, D9]TMPA 2 has no 1H within two bonds, which could explain the long T1 achieved18. However, unexpectedly, it was found that TMPA 2 was still affected by 15N–1H DD relaxation. The role of 15N–1H DD relaxation in total relaxation, termed 15N–1H DD relaxation%, can be discussed by measurement of nuclear Overhauser enhancement (NOE, 1 + η) as previously reported (equation 2)19,20,21,22. Such 15N–1H DD relaxation% indicates whether it is possible to elongate T1 value by reducing the DD relaxation. A 15N–1H DD relaxation% value of 89.6% was determined for [15N]TMPA 1 (Table 1), whereas for [15N, D9]TMPA 2, wherein all methyl 1H atoms were replaced with 2H atoms, 15N–1H DD relaxation was reduced but remained nonnegligible (21.0%). From these results, we expected that replacement of the aromatic 1H atoms with 2H, despite them being three or more bonds away from the 15N atom, could further extend the 15N T1 of the TMPA structure.

With the above considerations in mind, we then designed fully deuterated TMPA 3 ([15N, D14]TMPA, Fig. 1). TMPA 3 was synthesized from [D6]benzene in three steps: nitration with [15N]HNO3, reduction through Pd/C hydrogenation, and nucleophilic displacement with CD3I (Fig. 2).
The T1 value of TMPA 3 was extended compared with those of both TMPA 1 and 2 (Table 1). The 15N T1 of TMPA 3 was determined to be 1128 ± 112 s (14.1 T, 30 °C, D2O) and 1177 ± 52 s (9.4 T, 30 °C, D2O) by using the saturation recovery method under non-degassed condition. 15N–1H DD relaxation% of TMPA 3 was quite low (1.3%) and T1 observed was longer than 1000 s in D2O (Table 1). This increase in the T1 of TMPA 3 could be realized by minimizing 15N–1H DD relaxation in the fully deuterated [15N]TMPA.
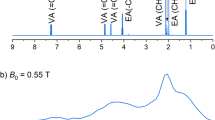
The long T1 value for TMPA 3 led to a long retention of the hyperpolarized spin state. TMPA 3 was applied for dynamic nuclear polarization process using HyperSense (Oxford Instruments; UK) and subjected to 15N NMR analysis after rapid dissolution. As shown in Fig. 3a, the 15N NMR signal was clearly observed by measuring a single scan (5° pulse angle). The signal was enhanced by 5700 times (%P15N = 1.9%, T = 298 K, B0 = 9.4 T) compared with that in the thermally equilibrated state. Importantly, the enhanced NMR signal of hyperpolarized 3 could be observed on a long time scale (stacked spectra in Fig. 3b), allowing detection of the hyperpolarized 15N signal for over 1 h under our experimental conditions (Fig. 3c). These results indicate that fully deuterated TMPA 3 has considerable potential for use as a long-lived hyperpolarization unit.
(a) Single-scan 15N NMR spectra of hyperpolarized and thermally equilibrated [15N, D14]TMPA 3 (27 mM). (b) 15N NMR spectra of hyperpolarized 3 (27 mM) stacked from 0 (90 s after dissolution) to 1.75 h (every 90 s, pulse angle 5°). (c) Decay of the 15N NMR signal of hyperpolarized [15N, D14]TMPA 3 (red circle) and theoretical signal decay estimated by the equation30 on each T1 value (gray dotted line) (every 90 s, pulse angle 5°). Experiments were conducted in D2O containing 0.025% EDTA disodium salt.
With TMPA 3 having a long 15N T1, we then conducted a proof-of-concept experiment by designing a long-lived hyperpolarized MR sensor.
The detection target was carboxylesterase (CE), which is a fundamental enzyme for ester hydrolysis. CE catalyzes phase I metabolism and a hydrolytic reaction by CE is used as an activation mechanism of several prodrugs23,24. Therefore, CE has been an attractive target of hyperpolarized MR molecular sensors25.
TMPA 3 was converted into CE sensor 4, wherein a deuterated methyl ester group was introduced at para position to the 15N nuclei (Fig. 4a). Compound 4 was synthesized from [D6]phenol in four steps (Supplementary Figure S1). The T1 values of sensor 4 were 795 ± 42 s (9.4 T, 30 °C, D2O) and 602 ± 52 s (9.4 T, 37 °C, 90% H2O + 10% D2O). These T1 values were longer than those of previously reported [15N, D9]TMPA-based esterase probe (Supplementary Figure S2; 570 ± 86 s, 9.4 T, 30 °C, D2O; 450 ± 15 s, 9.4 T, 37 °C, 90% H2O + 10% D2O) under the same experimental conditions18.
(a) Probe 4 for sensing of carboxylesterase actvity. (b) Stacked single-scan 15N NMR spectra of hyperpolarized probe 4 (13.3 mM, every 90 s, 5° pulse angle, 0 min = 90 s after dissolution) after mixing (left) with or (right) without esterase (25 units, derived from porcine liver) in phosphate-buffered saline (H2O, pH 7.4).
Hyperpolarized molecule 4 worked as a chemical shift-switching sensor for detection of CE activity in phosphate-buffered saline (H2O, pH 7.4) (Fig. 4a and b). The high sensitivity of the technique meant that 15N NMR spectra of hyperpolarized sensor 4 could be detected by a single-scan measurement (50.5 ppm). In the presence of CE derived from porcine liver, hyperpolarized sensor 4 afforded a new 15N signal 1.1 ppm upfield of the parent signal (49.4 ppm). This new peak was assigned as that of hydrolyzed product 4 by thermally equilibrated NMR analysis, suggesting that this hyperpolarized probe is suitable for use as a molecular sensor for CE activity. The 1.1 ppm change in 15N chemical shift may need to be enlarged especially for in vivo application26,27. However, it is sufficient to monitor enzymatic reaction in vitro. Thanks to the long T1, this enzymatic reaction could be tracked for approximately 40 min, which is longer than is possible for typical hyperpolarized molecular sensors (tens of seconds or a few minutes).
In summary, we have developed a molecular unit that is characterized by a remarkably long retention of hyperpolarized signal. The developed [15N, D14]TMPA unit showed a long T1 value (1128 s, 14.1 T, 30 °C, D2O) on its 15N nuclei and long-lived hyperpolarization. To our knowledge, this T1 is the longest among the soluble 13C or 15N small organic molecules utilized in hyperpolarization studies10,11,18. Such a long T1 value allows the molecule to be used in a diverse range of applications. [15N, D14]TMPA can work as a platform for designing a variety of hyperpolarized molecular sensors as shown in this study. Furthermore, it is feasible to use [15N, D14]TMPA as a hyperpolarized MRI tracer for post-polarization labelling28 of biomolecules such as peptides, proteins, and synthetic ligands, while conventional hyperpolarized units are not suitable for use in labelling techniques because of their limited hyperpolarization lifetimes. The [15N, D14]TMPA unit, in combination with a smart cross-polarization technique to boost the polarization level29, might realize such applications.
In this study, the long hyperpolarized lifetime was achieved by minimizing 15N-1H DD T1-relaxation, suggesting that it is possible to design a longer-lived hyperpolarization unit by carefully investigating T1 relaxation contributions. The knowledge and information obtained by these trials would provide important guidelines for the design of new hyperpolarized probes. Further work along these lines is under way in our laboratories.
Methods
General information on synthesis
Reagents and solvents were purchased from standard suppliers and used without further purification. Gel permeation chromatography (GPC) was performed on JAIGEL GS310 using a JAI Recycling Preparative HPLC LC-9201. NMR spectra were measured using a Bruker Avance III spectrometer (400 MHz for 1H) and a JEOL ECS400 spectrometer. Acetone-d6 in methanol (2.15 ppm) was used as the internal standard for 2H NMR. Chloroform-d1 (77.0 ppm) or methanol-d4 (49.0 ppm) was used as the internal standard for 13C NMR. Choline chloride-15N (43.4 ppm) was used as the external standard for 15N NMR. Mass spectra (MS) were measured using a JEOL JMS-HX110A (FAB).
Synthesis of [15N, D5]4-nitrobenzene
[15N]Nitric acid 40w/w% (3.00 mL, 23.8 mmol) was added dropwise to a solution of benzene-d6 (2.00 g, 23.8 mmol) in sulfuric acid (3.2 mL) on ice. The mixture was stirred at 50 °C for 1 h. Ice and water were added to the mixture. After removing the aqueous phase, the resulting organic phase was purified by distillation using Kugelrohr apparatus to give [15N, D5] 4-nitrobenzene as a pale yellow liquid (980 mg, 32%): 13C NMR (CDCl3, 100 MHz) δ = 122.7 (1JCD = 26 Hz), 128.5 (1JCD = 25 Hz), 133.9 (1JCD = 25 Hz), 147.7 (d, 1JCN = 15 Hz); 15N NMR (CDCl3, 40 MHz) δ = 366.5.
Synthesis of [15N, D14]trimethylphenylammonium (3)
[15N, D5]4-Nitrobenzene (500 mg) in methanol (5 mL) was stirred under hydrogen at r.t. for 2 h in the presence of 10 wt.% palladium on activated carbon (50 mg). The solution was filtered, and the filtrate was evaporated to give the reductant. Subsequently, [D3]iodomethane (938 μL, 15.1 mmol) was added to a solution of the residue (326 mg, 3.29 mmol) and N,N′-diisopropylethylamine (2.29 mL, 13.2 mmol) in dry DMF (7 mL). The mixture was stirred at room temperature for 35 h. After evaporation, EtOAc was added to the crude residue to produce a white precipitate. The resulting precipitate was filtered and purified using GPC (eluent: methanol) to give 3 as a white powder (235 mg, 22% for 2 steps): 2H NMR (MeOH, 61 MHz) δ = 3.7 (9 × 2H), 7.7 (2 × 2H + 1 × 2H), 8.0 (2 × 2H); 13C NMR (CD3OD, 100 MHz) δ = 55.2–56.1 (m), 119.6 (1JCD = 25 Hz), 129.7–130.1 (m), 147.0 (d, 1JCN = 7 Hz); 15N NMR (D2O, 40 MHz) δ = 50.9; HRMS(FAB): m/z calc. for C9D1415N [M−I]+ = 151.1975, found = 151.1971.
T1 measurements
All T1 measurements were performed at thermal equilibrium. The T1 measurements of MR probes were performed using a JEOL ECA 600 (14.1 T, 30 °C, 200–500 mM) and JEOL ECS 400 (9.4 T, 30 °C, 200–400 mM) by saturation recovery method under non-degassed condition.
NOE (1 + η) measurements
The NOE (1 + η) of MR probes 1–3 was measured on a JEOL ECS 400 (9.4 T, 30 °C). After measuring the 15N NMR spectra with or without NOE, the value η was determined using the following equation 3 19,20:

General information on DNP–NMR measurements
Tris{8-carboxyl-2,2,6,6-tetra[2-(1-hydroxyethyl)]-benzo(1,2-d:4,5-d′)bis(1,3)dithiole-4-yl}methyl sodium salt (Ox63 radical, GE Healthcare) and the 15N-labelled sample were dissolved in a 1:1 solution of D2O (99.9%, D):dimethyl sulfoxide-d6 (99.8%, D) (final concentration of Ox63 10–15 mM). The sample was submerged in liquid helium in a DNP polarizer magnet (3.35 T) (HyperSense, Oxford Instruments). The transfer of polarization from the electron spin on the radical to the 15N nuclear spin on the probe was achieved using microwave irradiation at 94 GHz and 100 mW under 2.8 mbar at 1.4 K. After polarization, samples were dissolved in D2O containing 0.025% EDTA disodium salt or an appropriate buffer heated to ~185 °C (pressurized to 10 bar)18. The DNP-NMR measurement was performed using a Japan Redox JXI-400Z spectrometer (9.4 T).
Time course analysis of hyperpolarized [15N, D14]TMPA (3)
The hyperpolarized [15N, D14]TMPA (final concentration 27 mM) was dissolved in D2O containing 0.025% EDTA disodium salt (4 mL). The solution was passed through two anion exchange cartridges (Grace: USA) to remove the remaining Ox63 radical, transferred to a 10 mm NMR tube, and subjected to 15N NMR analysis (flip angle 5°, repetition time 90 s).
Carboxyesterase sensing by probe 4
The hyperpolarized probe 4 (final concentration 13.3 mM) was dissolved in PBS (H2O, pH 7.4) containing 0.025% EDTA disodium salt (4 mL). The solution was passed through two anion exchange cartridges (Grace: USA) to remove the remaining Ox63 radical. The resulting solution was mixed with carboxylesterase (Sigma-Aldrich E2884, 25 units), transferred to a 10 mm NMR tube, and subjected to 15N NMR analysis (flip angle 5°, repetition time 90 s).
Additional Information
How to cite this article: Nonaka, H. et al. Design of a 15N Molecular Unit to Achieve Long Retention of Hyperpolarized Spin State. Sci. Rep. 7, 40104; doi: 10.1038/srep40104 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Lee, J. H., Okuno, Y. & Cavagnero, S. Sensitivity enhancement in solution NMR: Emerging ideas and new frontiers. J. Magn. Reson. 241, 18–31 (2014).
Kurhanewicz, J. et al. Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia 13, 81–97 (2011).
Nelson, S. J. et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci. Transl. Med. 5, 198ra108 (2013).
Chaumeil, M. M. et al. Non-invasive in vivo assessment of IDH1 mutational status in glioma. Nat. Commun. 4, 2429 (2013).
Nishihara, T. et al. Direct Monitoring of γ-Glutamyl Transpeptidase Activity In Vivo Using a Hyperpolarized 13C-Labeled Molecular Probe. Angew. Chem. Int. Ed. 55, 10626–10629 (2016).
Keshari, K. R., Kurhanewicz, J., Macdonald, J. M. & Wilson, D. M. Generating contrast in hyperpolarized 13C MRI using ligand–receptor interactions. Analyst 137, 3427–3429 (2012).
Chambers, J. M. et al. Cryptophane Xenon-129 Nuclear Magnetic Resonance Biosensors Targeting Human Carbonic Anhydrase. J. Am. Chem. Soc. 131, 563–569 (2009).
Lee, Y., Heo, G. S., Zeng, H., Wooley, K. L. & Hilty, C. Detection of Living Anionic Species in Polymerization Reactions Using Hyperpolarized NMR. J. Am. Chem. Soc. 135, 4636–4639 (2013).
Lelli, M. et al. Fast Characterization of Functionalized Silica Materials by Silicon-29 Surface-Enhanced NMR Spectroscopy Using Dynamic Nuclear Polarization. J. Am. Chem. Soc. 133, 2104–2107 (2011).
Keshari, K. R. & Wilson, D. M. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem. Soc. Rev. 43, 1627–1659 (2014).
Meier, S., Jensen, P. R., Karlsson, M. & Lerche, M. H. Hyperpolarized NMR Probes for Biological Assays. Sensors 14, 1576–1597 (2014).
Allouche-Arnon, H., Lerche, M. H., Karlsson, M., Lenkinski, R. E. & Katz-Brull, R. Deuteration of a molecular probe for DNP hyperpolarization – a new approach and validation for choline chloride. Contrast Media Mol. Imaging 6, 499–506 (2011).
Kumagai, K. et al. Synthesis and hyperpolarized 15N NMR studies of 15N-choline-d13 . Tetrahedron 69, 3896–3900 (2013).
Doura, T., Hata, R., Nonaka, H., Ichikawa, K. & Sando, S. Design of a 13C Magnetic Resonance Probe Using a Deuterated Methoxy Group as a Long-Lived Hyperpolarization Unit. Angew. Chem. Int. Ed. 51, 10114–10117 (2012).
Chiavazza, E., Viale, A., Karlsson, M. & Aime, S. 15N-Permethylated amino acids as efficient probes for MRI-DNP applications. Contrast Media Mol. Imaging 8, 417–421 (2013).
Rodrigues, T. B. et al. Magnetic resonance imaging of tumor glycolysis using hyperpolarized 13C-labeled glucose. Nat. Med. 20, 93–97 (2014).
Theis, T. et al. Direct and cost-efficient hyperpolarization of long-lived nuclear spin states on universal 15N2-diazirine molecular tags. Sci. Adv. 2, e1501438 (2016).
Nonaka, H. et al. A platform for designing hyperpolarized magnetic resonance chemical probes. Nat. Commun. 4, 2411 (2013).
Levy, G. C. Carbon-13 Spin-Lattice Relaxation Studies and Their Application to Organic Chemical Problems. Acc. Chem. Res. 6, 161–169 (1973).
Levy, G. C., Holloway, C. E., Rosanske, R. C., Hewitt, J. M. & Bradley, C. H. Natural Abundance Nitrogen-15 n.m.r. Spectroscopy. Spin-Lattice Relaxation in Organic Compounds. Org. Magn. Reson. 8, 643–647 (1976).
Saluvere, T. & Lippmaa, E. Spin-Lattice Relaxation Of 15N Nuclei. Chem. Phys. Lett. 7, 545–548 (1970).
Lippmaa, E., Saluvere, T. & Laisaar, S. Spin-Lattice Relaxation Of 15N Nuclei In Organic Compounds. Chem. Phys. Lett. 11, 120–123 (1971).
Hosokawa, M. Structure and Catalytic Properties of Carboxylesterase Isozymes Involved in Metabolic Activation of Prodrugs. Molecules 13, 412–431 (2008).
Rautio, J. et al. Prodrugs: design and clinical applications. Nat. Rev. Drug Discov. 7, 255–270 (2008).
Jensen, P. R. et al. Hyperpolarized [1,3-13C2]ethyl acetoacetate is a novel diagnostic metabolic marker of liver cancer. Int. J. Cancer. 136, E117–E126 (2015).
Jiang, W. et al. Hyperpolarized 15N-pyridine Derivatives as pH-Sensitive MRI Agents. Sci. Rep. 5, 9104 (2015).
Hata, R., Nonaka, H., Takakusagi, Y., Ichikawa, K. & Sando, S. Design of a hyperpolarized 15N NMR probe that induces a large chemical-shift change upon binding of calcium ions. Chem. Commun. 51, 12290–12292 (2015).
Wilson, D. M. et al. Generation of hyperpolarized substrates by secondary labeling with [1,1-13C] acetic anhydride. Proc. Natl. Acad. Sci. USA 106, 5503–5507 (2009).
Vuichoud, B. et al. Hyperpolarization of Deuterated Metabolites via Remote Cross- Polarization and Dissolution Dynamic Nuclear Polarization. J. Phys. Chem. B 118, 1411–1415 (2014).
Lumata, L. et al. DNP by Thermal Mixing under Optimized Conditions Yields > 60 000-fold Enhancement of 89Y NMR Signal. J. Am. Chem. Soc. 133, 8673–8680 (2011).
Acknowledgements
This work was supported by CREST, Japan Science and Technology Agency (JST). The use of HyperSense was in part supported by the funding program ‘Creation of Innovation Centers for Advanced Interdisciplinary Research Areas’ from JST. The authors thank Ms. Kaori Inoue of Kyushu University for her help in hyperpolarized-NMR measurements. We also thank the Network Joint Research Center for Materials and Devices for T1 and FAB–MS measurements. H.N. thanks the Takeda Science Foundation for financial support.
Author information
Authors and Affiliations
Contributions
H.N. and S.S. conceived the project and designed the experiments. H.N., M.H., and Y.I. performed all the experiments with help from Y.T. and K.I. on DNP and NMR measurements. The manuscript was written by H.N. and S.S. and edited by all the co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Nonaka, H., Hirano, M., Imakura, Y. et al. Design of a 15N Molecular Unit to Achieve Long Retention of Hyperpolarized Spin State. Sci Rep 7, 40104 (2017). https://doi.org/10.1038/srep40104
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep40104
This article is cited by
-
Hyperpolarized 15N-labeled, deuterated tris(2-pyridylmethyl)amine as an MRI sensor of freely available Zn2+
Communications Chemistry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.