Abstract
The spirochete bacterium Borrelia afzelii is the most common cause of Lyme borreliosis in Europe. This tick-borne pathogen can establish systemic infections in rodents but not in birds. However, several field studies have recovered larval Ixodes ricinus ticks infected with B. afzelii from songbirds suggesting successful transmission of B. afzelii. We reviewed the literature to determine which songbird species were the most frequent carriers of B. afzelii-infected I. ricinus larvae and nymphs. We tested experimentally whether B. afzelii is capable of co-feeding transmission on two common European bird species, the blackbird (Turdus merula) and the great tit (Parus major). For each bird species, four naïve individuals were infested with B. afzelii-infected I. ricinus nymphal ticks and pathogen-free larval ticks. None of the co-feeding larvae tested positive for B. afzelii in blackbirds, but a low percentage of infected larvae (3.33%) was observed in great tits. Transstadial transmission of B. afzelii DNA from the engorged nymphs to the adult ticks was observed in both bird species. However, BSK culture found that these spirochetes were not viable. Our study suggests that co-feeding transmission of B. afzelii is not efficient in these two songbird species.
Similar content being viewed by others
Introduction
The tick-borne spirochete bacterium Borrelia afzelii is the most common etiological agent of Lyme borreliosis (LB) in Europe1,2,3. This pathogen is transmitted by Ixodes ricinus ticks and is adapted to infect rodent reservoir hosts3,4,5,6,7. In these hosts, B. afzelii establishes a long-term, systemic infection that facilitates high rates of host-to-tick transmission6,8,9,10,11. In contrast to bird-adapted Borrelia species such as B. garinii and B. valaisiana, experimental infection studies with blackbirds, pheasants, and great tits have shown that B. afzelii is not able to establish a systemic infection in these bird species12,13,14. The ability of B. afzelii to infect rodent but not avian hosts (and vice versa for the bird-adapted Borrelia species) appears to be mediated by the vertebrate complement system15,16. Thus, the general consensus is that B. afzelii is unable to use avian hosts to infect new ticks1,2,3,17.
Recent field studies on birds have questioned this consensus of whether B. afzelii is strictly incompatible with avian hosts. Many species of birds are frequently exposed to B. afzelii-infected I. ricinus nymphs18,19,20,21,22,23,24. More importantly, B. afzelii-infected larval ticks have been recovered from a number of bird species including Fringilla coelebs L., Troglodytes troglodytes L., Parus major L., Turdus merula L., and Turdus iliacus L. (see Table 1). Given that vertical transmission of LB pathogens is thought to be rare in Ixodes ticks25,26,27, these observations suggest that these larval ticks acquired B. afzelii spirochetes from avian hosts.
Co-feeding transmission is one strategy by which B. afzelii might infect larval ticks feeding on avian hosts. This mode of transmission occurs when infected and uninfected ticks feed in close spatial and temporal proximity on the same host28,29,30. A number of studies have documented co-feeding transmission of B. afzelii on competent rodent reservoir hosts28,31,32,33,34. The observation that this mode of transmission can occur in the absence of a systemic infection raised the hypothesis that co-feeding transmission could allow Borrelia pathogens to evade the hostile immune system of otherwise incompetent hosts29,30,35. For example, co-feeding transmission of B. afzelii and B. garinii has been documented on ungulates, which are believed to be refractory to systemic infection36,37. An experimental infection study using a Japanese strain of B. garinii demonstrated co-feeding transmission on laboratory mice38. However, an alternative explanation for this study is that this strain actually belonged to the closely related but rodent-adapted B. bavariensis, as this species was recently shown to be widespread in Asia, including Japan39.
The purpose of the present study was to test whether B. afzelii can use co-feeding transmission to infect I. ricinus larval ticks on two different species of songbird: the blackbird (Turdus merula) and the great tit (Parus major). We chose these two songbird species because they are common in Europe, are often exposed to immature I. ricinus ticks in nature, and they are highly competent reservoir hosts for bird-adapted Borrelia genospecies. The blackbird can amplify B. garinii, B. valaisiana and B. turdi24,40,41 and the great tit can amplify B. garinii12. In addition, we performed a literature review to determine how often songbirds carry B. afzelii-infected immature I. ricinus ticks in nature.
Results
Blackbird experiment
In the blackbird experiment, each of the four birds was infested with 11–12 nymphs before being infested with 40–50 co-feeding larvae 24 hours later. The challenge nymphs had been randomly selected from a population where the percentage of infected nymphs was 68.1% (47 infected/69 total). For the blackbirds, the nymphal and larval attachment rates (mean ± standard deviation) were 93.7 ± 12.5% per bird and 96.5 ± 4.7% per bird, respectively. A total of 20 engorged challenge nymphs and 128 engorged co-feeding larvae were recovered (mean ± standard deviation: 5.0 ± 0.8 nymphs per bird and 32 ± 12 larvae per bird). The engorged challenge nymphs were allowed to moult into adult ticks, which were tested using qPCR to determine whether the birds had been exposed to B. afzelii. A total of 17 challenge nymphs and 90 co-feeding larvae were tested for the four blackbirds (Table 2).
Two of the four blackbirds produced 2 and 4 infected adult ticks (Table 2) indicating that they were properly challenged. The presence of B. afzelii in 6 adult ticks suggests that there was nymph-to-adult transtadial transmission but we do not know whether these spirochetes were dead or alive. The other two birds produced 2 and 4 uninfected adult ticks (Table 2). Given that the estimated proportion of infected challenge nymphs was 0.681, the probability that these two birds would produce 6 uninfected adult ticks is (1–0.681)6 = 0.001. Our method of estimating nymphal attachment suggests that 9 and 11 challenge nymphs attached to these two birds. The probability that these two birds were infested with at least one B. afzelii-infected nymph is therefore very high (0.9999659 and 0.9999965, respectively). Thus we are confident that all four birds encountered at least one B. afzelii-infected nymph. However, none of the 90 xenodiagnostic larval ticks (tested as engorged larvae or as flat nymphs) that had co-fed with the challenge nymphs tested positive for B. afzelii (Table 2).
All ticks that had fed on the blackbirds and that had tested positive for B. afzelii on the qPCR were sequenced with respect to the ospC gene and the 5S-23S (rrfA-rrlB) intergenic spacer (IGS) region gene. We obtained 3 ospC sequences and 5 IGS sequences and all of them belonged to B. afzelii. This sequencing work confirms that the nymphs used to challenge the blackbirds were infected with B. afzelii.
Great tit experiment
In the great tit experiment, each of the four birds was infested with 11–12 nymphs before being infested with 40–50 co-feeding larvae 24 hours later. The challenge nymphs had been randomly selected from a population where the percentage of infected nymphs was 91.5% (130 infected/142 total). For the great tits, the nymphal and larval attachment rates (mean ± standard deviation) were 58.3 ± 21.5% per bird and 85.6 ± 9.8% per bird, respectively. A total of 16 engorged challenge nymphs and 115 engorged co-feeding larvae were recovered (mean ± standard deviation: 4.0 ± 2.7 nymphs per bird and 28.8 ± 6.8 larvae per bird). The engorged challenge nymphs were either tested directly or were allowed to moult into adult ticks. A total of 16 challenge nymphs and 90 co-feeding larvae were tested for the four great tits (Table 2).
Analysis of the challenge ticks showed that all four great tits had been exposed to B. afzelii (2, 3, 8, and 2 infected ticks per bird; Table 2). Three of the 76 xenodiagnostic larval ticks (tested as engorged larvae) that had co-fed with the challenge nymphs tested positive for B. afzelii, but the pathogen was not detected in any of the 14 nymphs (moulted from the engorged larvae) (Table 2). Four of the five adult ticks obtained from three birds tested positive for B. afzelii based on the qPCR (Table 2), but the culture of these ticks in BSK-II medium did not yield any viable spirochetes.
Summary of the infection experiments
Overall, the B. afzelii-infection rates in co-feeding larvae were low in both blackbirds (0.00% = 0/90) and great tits (3.33% = 3/90). In summary, we found limited co-feeding transmission of B. afzelii for the two bird species used in this study. We emphasize that our sample size was limited with only 4 individuals for each bird species.
Literature review
Our review of the literature found 13 of 19 studies in which B. afzelii has been reported in songbird-derived I. ricinus ticks. Seven species of songbird could play a role in the transmission of B. afzelii to larval I. ricinus ticks (Table 1). The hosts that were most often reported to have B. afzelii-infected larvae were the European robin (Erithacus rubecula) and the great tit (2 studies). When considering birds that carried B. afzelii-infected nymphal ticks, we found 20 different bird species, of which the blackbird (7 studies), songthrush (Turdus philomenos) (6 studies), dunnock (Prunella modularis) (5 studies), European robin (5 studies), and great tit (4 studies) were most often reported.
Discussion
Our study suggests that the rodent-adapted Lyme disease pathogen, B. afzelii, cannot use co-feeding transmission as an efficient strategy to infect naive ticks on two species of songbird. There was no co-feeding transmission of B. afzelii on the four blackbirds and only three larval ticks acquired B. afzelii via co-feeding transmission on the four great tits. The efficiency of co-feeding transmission of B. afzelii on the great tit was therefore low (3/90 = 3.33%). In contrast, the isolate of B. afzelii used in the great tit experiment (isolate NE4049; also referred to as ospC strain A10) has high co-feeding transmission (> 50.00%) on competent rodent reservoir hosts, and in these hosts there is successful trans-stadial transmission32,34. We acknowledge that one limitation of the current study is the small sample size (n = 8 birds). However, we point out that studies with similar sample sizes have detected co-feeding transmission of B. afzelii on rodents28,32,33. Recent theoretical studies have shown that co-feeding transmission makes a modest contribution to the reproductive number (R0) of B. burgdorferi pathogens42,43,44. Specifically, a co-feeding transmission efficiency of 50.0% increases the R0 value by 2.07–6.68% depending on a variety of ecological factors42. These analyses suggest that a co-feeding transmission efficiency of 3.33% would have a negligible effect on the R0 of B. afzelii. In summary, B. afzelii is transmitted efficiently via co-feeding transmission on rodent hosts but not on the two bird species investigated. Studies on B. afzelii in laboratory rodents have shown that strains differ in the efficacy of co-feeding transmission32,34. Studies on B. burgdorferi in North American passerines have shown that reservoir competence can vary widely between bird species45,46,47. We therefore emphasize that we cannot generalize these results to other strains of B. afzelii and other songbird species.
Our study also found evidence that avian blood is borreliacidal for B. afzelii. For the blackbirds, the probability that two birds would produce six uninfected adult ticks was highly unlikely (p = 0.001), given that an independent sample suggested that 68.1% (47 infected/69 total) of the challenge nymphs were infected with B. afzelii before feeding on these birds. Our results are similar to a previous study where B. afzelii was cleared from I. ricinus challenge nymphs after they had fed on pheasants, whereas bird-adapted Borrelia species were not cleared from the challenge nymphs12. Additional evidence for the borreliacidal effects of avian blood on B. afzelii was our demonstration using BSK-II cultures that none of the qPCR-positive adult ticks that had fed as nymphal ticks on the great tits contained viable spirochetes. Previous work has shown that the ability to detect Borrelia infections by culturing ticks in BSK media is similar to PCR-based methods48. This result suggests that our qPCR assay is detecting dead spirochetes in the adult ticks and shows the limitations of using DNA-based methods to infer the reservoir competence of a particular host species. Further studies using other combinations of pathogen strains and songbird species should investigate the generality of whether avian blood kills B. afzelii in I. ricinus during tick blood feeding.
Numerous field studies have shown the association of B. afzelii with rodent reservoir hosts4,5,6,49,50 and of B. garinii and B. valaisiana with avian reservoir hosts7,12,13,21,24,40,41,51,52,53. The cycling of B. afzelii and B. garinii in different classes of vertebrate hosts is also supported by studies on wild I. ricinus nymphs, which have shown that these two sympatric Borrelia species rarely co-occur in the same nymphal tick54,55,56. The host-specificity of B. afzelii for rodents and B. garinii for birds is believed to be mediated by the complement system of the vertebrate host15,16,55,56. In vitro assays have shown that B. afzelii is tolerant to rodent complement but is lysed by bird complement, and vice versa for bird-adapted Borrelia species like B. garinii and B. valaisiana15,16. However, as mentioned previously, there are very few in vivo studies showing that B. afzelii spirochetes are killed in nymphs that feed on avian hosts13. Two recent studies that quantified the abundance of rodent- and bird-adapted Borrelia species in wild questing I. ricinus nymphs provided indirect evidence for the complement hypothesis54,57. In the first study, the spirochete load of nymphs co-infected with rodent- and bird-adapted Borrelia species was significantly lower than the additive expectation of when the species occurred alone54. In the second study, co-infections between B. afzelii and B. garinii were surprisingly common in wild nymphs, however, the spirochete load of the dominant Borrelia species was always an order of magnitude higher than the sub-dominant species57. Taken together, these two studies provide indirect evidence that some component of the vertebrate blood meal (e.g. complement) was reducing the spirochete load of the mal-adapted Borrelia species54,57. Thus co-infections between rodent- and bird-adapted Borrelia species in I. ricinus nymphs may be much more common than previously thought but the spirochete population of one of the two species is probably dead.
Migratory songbirds have a great capacity to disperse ticks and tick-borne pathogens to new geographic locations58. Interestingly, phylogenetic studies have shown that B. afzelii has much more spatial genetic structure than B. garinii, which may reflect the migratory potential of their rodent and bird hosts59,60. Our literature review found that ground-dwelling birds such as the blackbird, song thrush, European robin and dunnock were common carriers of B. afzelii-infected immature I. ricinus ticks. These studies have led to speculation that B. afzelii can use bird hosts to achieve transmission and is not as restricted to rodent hosts as previously thought61. However, all of these studies used PCR-based methods to determine Borrelia infection and none of these studies used culture-based methods to show that the spirochetes are actually alive. The present study shows that nymph-to-adult transtadial transmission of B. afzelii DNA can occur on birds but that the spirochetes are not necessarily viable. We suggest that PCR-based studies demonstrating that birds can amplify B. afzelii or that rodents can amplify B. garinii should be interpreted with great caution.
We propose three alternative explanations for the observation that B. afzelii-positive larval ticks are regularly collected from wild birds (Table 1). First, the larval ticks could have acquired B. afzelii via vertical transmission. There is a general consensus that vertical transmission in Ixodes ticks is rare for B. burgdorferi s. l. pathogens but common for the relapsing fever spirochete B. miyamotoi25,26. A second explanation is partial blood feeding where larval ticks take multiple meals from different vertebrate hosts. Host blood meal analysis of wild I. ricinus ticks in Switzerland suggests that 9.5–19.5% of larval ticks feed on multiple hosts62,63. An early study on B. burgdorferi s. s. in I. scapularis showed that partially fed larval ticks could acquire spirochetes64. Thus larval ticks could acquire B. afzelii from a partial blood meal on a rodent and then attach to a bird to feed to repletion. A recent study in the Netherlands reported that wild I. ricinus larvae carried B. afzelii (prevalence was 0.62%), and these larvae were able to infect pathogen-free rodents27. The authors suggested that their data were consistent with both vertical transmission and partial blood meals27. A third explanation involves variation in the efficiency of co-feeding transmission between strains of B. afzelii. Like many vector-borne pathogens, populations of B. afzelii consist of multiple strains57,65,66,67,68. Two recent studies found that some B. afzelii strains are much more efficient at co-feeding transmission than other strains32,34. The B. afzelii strains in the blackbird experiment were derived from naturally infected Apodemus mice, and their genetic identity and co-feeding transmission efficiency on rodent hosts are currently unknown. For this reason, we used B. afzelii isolate NE4049 in the great tit experiment because it has a high efficiency of co-feeding transmission (>50%) on lab mice32,34.
We conclude that blackbirds and great tits do not allow efficient co-feeding transmission of viable B. afzelii spirochetes. The present study supports the hypothesis that the bird complement system inhibits the rodent-adapted B. afzelii from exploiting avian hosts for spirochete transmission. The generality of our results for other combinations of B. afzelii strains and bird species remains to be investigated.
Methods
Birds
Eurasian blackbirds and great tits are two abundant bird species in Europe. The Eurasian blackbird is frequently infested with tens of immature I. ricinus ticks24,69,70. The great tits in our Belgian study population frequently carry high burdens of immature I. ricinus ticks (maximum number of larvae = 40; nymphs = 17)71,72. Both bird species are competent reservoir hosts for bird-adapted B. burgdorferi s. l. pathogens. Blackbirds transmit B. garinii, B. valaisiana, and B. turdi24,40,41, whereas great tits transmit B. garinii12,73,74,75.
Four pathogen-free blackbirds and four pathogen-free great tits were obtained, respectively, from a certified Belgian breeder and a laboratory colony at the Netherlands Institute of Ecology (NIOO-KNAW)76. Environmental conditions consisted of a 12 h light: 12 h dark cycle (7:00 to 19:00) and ambient temperature varied with outdoor conditions. Birds were given food and water ad libitum, and had access to a fresh water bath. Birds were kept in individual cages and were allowed to habituate to the lab environment for at least four days before the start of the experiment.
Ixodes ricinus ticks
Pathogen-free I. ricinus larval ticks from the laboratory colony at the University of Neuchâtel were fed on B. afzelii-infected rodents and were allowed to moult into B. afzelii-infected nymphs (hereafter referred to as the challenge nymphs). The creation of the challenge nymphs was different for the blackbirds and great tits (see below). The pathogen-free I. ricinus larvae that were used for co-feeding with the infected challenge nymphs were obtained from a German laboratory colony (IS Insect Services GmbH, Berlin).
For the blackbirds, the challenge nymphs had been fed as larval ticks on 7 field-captured and naturally infected wood mice (Apodemus sylvaticus L.). Infection with B. burgdorferi s. l. of each wood mouse was confirmed with a commercial Lyme borreliosis ELISA assay and qPCR on an ear tissue sample, using protocols described elsewhere77. All challenge nymphs were kept in individual Eppendorf tubes to facilitate random sampling. We randomly selected 9–10 nymphs from each of the 7 Apodemus mice and tested them for B. afzelii infection using qPCR. The infection prevalence of the challenge nymphs used in the black bird experiment was 68.1% (47 infected/69 total).
For the great tits, the challenge nymphs had been fed as larval ticks on 15 Mus musculus BALB/c mice that had been experimentally co-infected via tick bite with B. afzelii isolates NE4049 and Fin-Jyv-A3. Infection with B. afzelii of each mouse was confirmed with a commercial Lyme borreliosis ELISA assay and qPCR on an ear tissue sample, using protocols described elsewhere77. Isolates Fin-Jyv-A3 and NE4049 were obtained from a bank vole (Myodes glareolus) in Finland and an I. ricinus nymph in Switzerland. Isolate Fin-Jyv-A3 carries ospC major group (oMG) A3. Isolate NE4049 has multi-locus sequence type 679, oMG A10, and strain ID number 1887 in the Borrelia MLST database11,32,34,77. We used isolate NE4049 (also referred to as ospC strain A10) because it has very efficient co-feeding transmission in lab mice11,32,34. All challenge nymphs were kept in individual Eppendorf tubes to facilitate random sampling. We randomly selected 7–10 nymphs from each of the 15 mice and tested them for B. afzelii infection using a previously described qPCR protocol77. The infection prevalence of the challenge nymphs used in the great tit experiment was 91.5% (130 infected/142 total), of which 75.4% (107/142) and 59.9% (85/142) were infected with isolates NE4049 and Fin-Jyv-A3, respectively.
Ethics statement and animal experimentation permits
Experiments on the birds were carried out at the University of Antwerp, Belgium in accordance with national environmental legislation and university regulations. The Ethics Committee for Animal Experiments of the University of Antwerp approved the tick infestation procedure (Dossier 2009-32) and the transmission experiment (Dossier 2014-49). Experiments to create the I. ricinus nymphs infected with B. afzelii were carried out at the University of Neuchâtel, Switzerland. The commission that is part of the ‘Service de la Consommation et des Affaires Vétérinaires (SCAV)’ of Canton Vaud, Switzerland evaluated and approved the ethics of this part of the study. The Veterinary Service of the Canton of Neuchâtel, Switzerland issued the animal experimentation permits (NE1/2014 and NE4/2016).
Study design
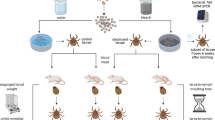
The infestation experiments for the blackbirds and great tits were conducted in November 2015 and February 2016, respectively. For each bird species, four individuals were infested with 11–12 B. afzelii-infected I. ricinus nymphs that had been randomly selected from the pool of available nymphs. These tick loads are within the range observed in field-captured birds24,69,70,71,72. Nymphs were placed underneath the crown feathers on the right side of the head above the eye using moistened tweezers, as described in ref. 72 (Fig. 1). After each infestation, birds were kept for 1 h in an air-permeable cotton bag (size: 25 cm × 20 cm for blackbirds; 20 cm × 15 cm for great tits) inside a darkened cage to keep them inactive and to facilitate tick attachment72. Twenty-four hours after nymphal exposure, the blackbirds and great tits were additionally infested with 40–50 xenodiagnostic larvae, following the same protocol as for the challenge nymphs. The larvae were placed near the nymphs to facilitate co-feeding transmission32,33,34. After each infestation, the cotton bags were checked for ticks to determine the number of nymphs and larvae that had attached to each bird. Birds were not checked for the number of attached nymphs to avoid disturbing these ticks. Following infestation, birds were returned to their individual cages (40 cm × 80 cm) that had a wire mesh floor to facilitate the daily collection of engorged ticks. Most of the engorged ticks were placed in 80% ethanol and stored at −20 °C. The remaining engorged ticks were allowed to moult to the next stage to study transstadial transmission of B. afzelii DNA. These ticks were kept in individual tubes under summer conditions (16 h light at 25 °C, 8 h at dark at 16 °C) and with a relative humidity >90%. For the great tit experiment, we further tested whether the B. afzelii spirochetes in the adult ticks were actually viable. Each of five adult ticks that had fed as challenge nymph on three great tits, were cut into two halves using sterile scissors. One tick half was screened for B. afzelii infection using qPCR, the other tick half was cultured in tubes containing BSK-II medium78, incubated at 34 °C, and examined by dark-field microscopy every 10 days for 40 days.
The larvae (small) and nymphs (large) were placed underneath the crown-feathers on the right side of the head (A: lateral; B: frontal view). By feeding in close spatial and temporal proximity, the B. afzelii spirochetes can migrate directly from the infected nymphs to the naïve larvae via co-feeding transmission. Dr. Frank Adriaensen took the photos.
Probability that each bird was challenged by at least one B. afzelii -infected nymph
If avian blood clears spirochetes from feeding nymphs, the post-hoc analysis of such ticks is not a reliable indicator as to whether the bird was challenged or not. For example, after feeding B. afzelii-infected I. ricinus nymphs on pheasants, 0 of the 56 engorged nymphs tested positive for B. afzelii13. In this case, it is critical to know the prevalence of B. afzelii infection in the flat nymphs (q) before they are placed on the birds, and the number of nymphs that attached to the bird (n). With this information one can calculate the probability (P) that each bird was bitten by at least one B. afzelii-infected challenge nymph as follows: P = 1 − (1 − q)n. The exact value of n is often unknown: the maximum is the number of nymphs that attached to the bird (nmax) and the minimum is the number of blood-engorged nymphs that were recovered (nmin). For example, for a bird that was infested with 12 challenge nymphs with an expected prevalence of infection of 0.681 and for which 4 engorged challenge nymphs were recovered, the probability that at least one of the challenge nymphs was infected with B. afzelii ranges from Pmax = 0.9999989 to Pmin = 0.9896447.
PCR-based detection of B. afzelii
Total tick DNA was purified using the DNeasy Blood & Tissue Kit following the protocol for the purification of total DNA from ticks. All ticks were screened for the presence of B. burgdorferi s. l. using a duplex qPCR that was designed based on existing qPCR protocols that target fragments of the ospA gene79 and the flagellin gene80. A detailed description of primers, probes and the qPCR protocol is given in an earlier study75. For the subsample of qPCR-positive ticks that had fed on the blackbirds, the B. burgdorferi s. l. genospecies was determined by PCR amplification and sequencing of the ospC gene81 and the variable 5S-23S (rrfA-rrlB) intergenic spacer (IGS) region gene75. For each PCR and multiplex qPCR, positive controls, negative controls, and blank samples were included. To minimize contamination, the three steps of the PCR protocol were performed in separate rooms. The DNA extraction room was kept at negative pressure, whereas the reagent setup and sample addition rooms were kept at positive pressure. All rooms had airlocks.
Literature review
We used an extensive systematic literature search that is described in Hofmeester et al. (2016)82. The search strings and selection procedure as well as the dataset are provided in the supplementary material of that study (URL: http://iopscience.iop.org/article/10.1088/1748-9326/11/4/043001/meta). The search was done using PubMed, Web of Science and Scopus to review the occurrence of B. burgdorferi s. l. pathogens in Europe, in songbird hosts and their I. ricinus ticks. The last literature search was carried out in January 2015 and used the years 1945–2014. We added one more study to that dataset22. Only studies that identified the Borrelia genospecies in infected larvae and nymphs derived from songbirds were included, which resulted in 19 usable studies.
Additional Information
How to cite this article: Heylen, D. J. A. et al. Inefficient co-feeding transmission of Borrelia afzelii in two common European songbirds. Sci. Rep. 7, 39596; doi: 10.1038/srep39596 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Piesman, J. & Gern, L. Lyme borreliosis in Europe and North America. Parasitology 129, S191–S220 (2004).
Kurtenbach, K. et al. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat. Rev. Microbiol. 4 (2006).
van Duijvendijk, G., Sprong, H. & Takken, W. Multi-trophic interactions driving the transmission cycle of Borrelia afzelii between Ixodes ricinus and rodents: a review. Parasit. Vectors 8, 1–11 (2015).
Hanincova, K. et al. Association of Borrelia afzelii with rodents in Europe. Parasitology 126, 11–20 (2003).
Hu, C. M., Humair, P. F., Wallich, R. & Gern, L. Apodemus sp. rodents, reservoir hosts for Borrelia afzelii in an endemic area in Switzerland. Zbl. Bakt.-Int. J. Med. M. 285, 558–564 (1997).
Humair, P. F., Rais, O. & Gern, L. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: differential transmission pattern and overwintering maintenance. Parasitology 118, 33–42 (1999).
Kurtenbach, K. et al. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl. Environ. Microbiol. 64, 1169–1174 (1998).
Gern, L. et al. Borrelia burgdorferi in rodents (Apodemus flavicollis and A. sylvaticus): Duration and enhancement of infectivity for Ixodes ricinus ticks. Eur. J. Epidemiol. 10, 75–80 (1994).
Talleklint, L. & Jaenson, T. G. T. Is the small mammal (Clethrionomys glareolus) or the tick vector (Ixodes ricinus) the primary overwintering reservoir for the Lyme borreliosis spirochete in Sweden. J. Wildl. Dis. 31, 537–540 (1995).
Richter, D., Klug, B., Spielman, A. & Matuschka, F. R. Adaptation of diverse Lyme disease spirochetes in a natural rodent reservoir host. Infect. Immun. 72, 2442–2444 (2004).
Jacquet, M., Margos, G., Fingerle, V. & Voordouw, M. J. Comparison of the lifetime host-to-tick transmission between two strains of the Lyme disease pathogen Borrelia afzelii. Parasites & Vectors. (in press).
Heylen, D., Matthysen, E., Fonville, M. & Sprong, H. Songbirds as general transmitters but selective amplifiers of Borrelia burgdorferi sensu lato genotypes in Ixodes rinicus ticks. Environ. Microbiol. 16, 2859–2868 (2014).
Kurtenbach, K. et al. Differential survival of Lyme borreliosis spirochetes in ticks that feed on birds. Infect. Immun. 70, 5893–5895 (2002).
Matuschka, F. R. & Spielman, A. Loss of Lyme disease spirochetes from Ixodes ricinus ticks feeding on European blackbirds. Exp. Parasitol. 74, 151–158 (1992).
Kurtenbach, K. et al. Host association of Borrelia burgdorferi sensu lato - the key role of host complement. Trends Microbiol. 10, 74–79 (2002).
Kurtenbach, K., Sewell, H. S., Ogden, N. H., Randolph, S. E. & Nuttall, P. A. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 66, 1248–1251 (1998).
Humair, P. F. & Gern, L. The wild hidden face of Lyme borreliosis in Europe. Microbes Infect. 2, 915–922 (2000).
Comstedt, P. et al. Migratory passerine birds as reservoirs of Lyme borreliosis in Europe. Emerg. Infect. Dis. 12, 1087–1095 (2006).
Dubska, L. et al. Synanthropic birds influence the distribution of Borrelia species: analysis of Ixodes ricinus ticks feeding on passerine birds. Appl. Environ. Microbiol. 77, 1115–1117 (2011).
Geller, J. et al. Tick-borne pathogens in ticks feeding on migratory passerines in western part of Estonia. Vector-Borne Zoonot. 13, 443–448 (2013).
Hanincova, K. et al. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl. Environ. Microbiol. 69, 2825–2830 (2003).
Hasle, G., Bjune, G. A., Midthjell, L., Roed, K. H. & Leinaas, H. P. Transport of Ixodes ricinus infected with Borrelia species to Norway by northward-migrating passerine birds. Ticks Tick Borne Dis. 2, 37–43 (2011).
Kipp, S., Goedecke, A., Dorn, W., Wilske, B. & Fingerle, V. Role of birds in Thuringia, Germany, in the natural cycle of Borrelia burgdorferi sensu lato, the Lyme disease spirochaete. Int. J. Med. Microbiol. 296, 125–128 (2006).
Taragel’ová, V. et al. Blackbirds and song thrushes constitute a key reservoir of Borrelia garinii, the causative agent of borreliosis in Central Europe. Appl. Environ. Microbiol. 74, 1289–1293 (2008).
Richter, D., Debski, A., Hubalek, Z. & Matuschka, F. R. Absence of Lyme disease spirochetes in larval Ixodes ricinus ticks. Vector-Borne Zoonot. 12, 21–27 (2012).
Rollend, L., Fish, D. & Childs, J. E. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: A summary of the literature and recent observations. Ticks Tick Borne Dis. 4, 46–51 (2013).
van Duijvendijk, G. et al. Larvae of Ixodes ricinus transmit Borrelia afzelii and B. miyamotoi to vertebrate hosts. Parasit. Vectors 9, 1–7 (2016).
Gern, L. & Rais, O. Efficient transmission of Borrelia burgdorferi between cofeeding Ixodes ricinus ticks (Acari: Ixodidae). J. Med. Entomol. 33, 189–192 (1996).
Randolph, S. E., Gern, L. & Nuttall, P. A. Co-feeding ticks: epidemiological significance for tick-borne pathogen transmission. Parasitol. Today 12, 472–479 (1996).
Voordouw, M. J. Co-feeding transmission in Lyme disease pathogens. Parasitology 142, 290–302 (2015).
Hu, C. M. et al. Early detection of Borrelia burgdorferi sensu lato infection in Balb/c mice by co-feeding Ixodes ricinus ticks. Int. J. Med. Microbiol. 293, 421–426 (2003).
Jacquet, M., Durand, J., Rais, O. & Voordouw, M. J. Strain-specific antibodies reduce co-feeding transmission of the Lyme disease pathogen, Borrelia afzelii. Environ. Microbiol. 18, 833–845 (2016).
Richter, D., Allgower, R. & Matuschka, F. R. Co-feeding transmission and its contribution to the perpetuation of the Lyme disease spirochete Borrelia afzelii . Emerg. Infect. Dis. 8, 1421–1425 (2002).
Tonetti, N., Voordouw, M. J., Durand, J., Monnier, S. & Gern, L. Genetic variation in transmission success of the Lyme borreliosis pathogen Borrelia afzelii . Ticks Tick Borne Dis. 6, 334–343 (2015).
Gern, L. et al. European reservoir hosts of Borrelia burgdorferi sensu lato. Zbl. Bakt.-Int. J. Med. M. 287, 196–204 (1998).
Kimura, K. et al. Detection of Lyme disease spirochetes in the skin of naturally infected wild sika deer (Cervus nippon yesoensis) by PCR. Appl. Environ. Microbiol. 61, 1641–1642 (1995).
Ogden, N. H., Nuttall, P. A. & Randolph, S. E. Natural Lyme disease cycles maintained via sheep by cofeeding ticks. Parasitology 115, 591–599 (1997).
Sato, Y. & Nakao, M. Transmission of the Lyme disease spirochete, Borrelia garinii, between infected and uninfected immature Ixodes persulcatus during cofeeding on mice. J. Parasitol. 83, 547–550 (1997).
Margos, G. et al. Borrelia bavariensis sp nov is widely distributed in Europe and Asia. Int. J. Syst. Evol. Microbiol. 63, 4284–4288 (2013).
Humair, P. F., Postic, D., Wallich, R. & Gern, L. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochetes. Zbl. Bakt.-Int. J. Med. M. 287, 521–538 (1998).
Norte, A. C., de Carvalho, I. L., Nuncio, M. S., Ramos, J. A. & Gern, L. Blackbirds Turdus merula as competent reservoirs for Borrelia turdi and Borrelia valaisiana in Portugal: evidence from a xenodiagnostic experiment. Environ. Microbiol. Rep. 5, 604–607 (2013).
Harrison, A. & Bennett, N. The importance of the aggregation of ticks on small mammal hosts for the establishment and persistence of tick-borne pathogens: an investigation using the R-0 model. Parasitology 139, 1605–1613 (2012).
Harrison, A., Montgomery, W. I. & Bown, K. J. Investigating the persistence of tick-borne pathogens via the R-0 model. Parasitology 138, 896–905 (2011).
Hartemink, N. A., Randolph, S. E., Davis, S. A. & Heesterbeek, J. A. P. The basic reproduction number for complex disease systems: Defining R-0 for tick-borne infections. Am. Nat. 171, 743–754 (2008).
Ginsberg, H. S. et al. Reservoir competence of native north American birds for the Lyme disease spirochete, Borrelia burgdorferi. J. Med. Entomol. 42, 445–449 (2005).
Mather, T. N., Telford, S. R., Maclachlan, A. B. & Spielman, A. Incompetence of catbirds as reservoirs for the Lyme disease spirochete (Borrelia burgdorferi). J. Parasitol. 75, 66–69 (1989).
Richter, D., Spielman, A., Komar, N. & Matuschka, F. R. Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 6, 133–138 (2000).
Morán Cadenas, F. et al. A comparison of two DNA extraction approaches in the detection of Borrelia burgdorferi sensu lato from live Ixodes ricinus ticks by PCR and reverse line blotting. Vector-Borne Zoonot. 7, 555–561 (2007).
Humair, P. F. & Gern, L. Relationship between Borrelia burgdorferi sensu lato species, red squirrels (Sciurus vulgaris) and Ixodes ricinus in enzootic areas in Switzerland. Acta Trop. 69, 213–227 (1998).
Humair, P. F., Péter, O., Wallich, R. & Gern, L. Strain variation of Lyme disease spirochetes isolated from Ixodes ricinus ticks and rodents collected in two endemic areas in Switzerland. J. Med. Entomol. 32, 433–438 (1995).
Norte, A. C., Ramos, J. A., Gern, L., Nuncio, M. S. & Lopes de Carvalho, I. Birds as reservoirs for Borrelia burgdorferi s.l. in Western Europe: circulation of B. turdi and other genospecies in bird-tick cycles in Portugal. Environ. Microbiol. 15, 386–397 (2013).
Poupon, M. A. et al. Prevalence of Borrelia burgdorferi sensu lato in ticks collected from migratory birds in Switzerland. Appl. Environ. Microbiol. 72, 976–979 (2006).
Lommano, E., Dvorak, C., Vallotton, L., Jenni, L. & Gern, L. Tick-borne pathogens in ticks collected from breeding and migratory birds in Switzerland. Ticks Tick Borne Dis. 5, 871–882 (2014).
Herrmann, C., Gern, L. & Voordouw, M. Species co-occurrence patterns among Lyme borreliosis pathogens in the tick vector Ixodes ricinus . Appl. Environ. Microbiol. 79, 7273–7280 (2013).
Kurtenbach, K. et al. Distinct combinations of Borrelia burgdorferi sensu lato genospecies found in individual questing ticks from Europe. Appl. Environ. Microbiol. 67, 4926–4929 (2001).
Rauter, C. & Hartung, T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Appl. Environ. Microbiol. 71, 7203–7216 (2005).
Durand, J. et al. Cross-immunity and community structure of a multiple-strain pathogen in the tick vector. Appl. Environ. Microbiol. 81, 7740–7752 (2015).
Hasle, G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front. Cell. Infect. Microbiol. 3 (2013).
Vollmer, S. A. et al. Host migration impacts on the phylogeography of Lyme Borreliosis spirochaete species in Europe. Environ. Microbiol. 13, 184–192 (2011).
Vollmer, S. A. et al. Spatial spread and demographic expansion of Lyme borreliosis spirochaetes in Eurasia. Infect. Genet. Evol. 14, 147–155 (2013).
Franke, J., Moldenhauer, A., Hildebrandt, A. & Dorn, W. Are birds reservoir hosts for Borrelia afzelii? Ticks Tick Borne Dis. 1, 109–112 (2010).
Humair, P.-F. et al. Molecular identification of bloodmeal source in Ixodes ricinus ticks using 12S rDNA as a genetic marker. J. Med. Entomol. 44, 869–880 (2007).
Morán Cadenas, F. M. et al. Identification of host bloodmeal source and Borrelia burgdorferi sensu lato in field-collected Ixodes ricinus ticks in Chaumont (Switzerland). J. Med. Entomol. 44, 1109–1117 (2007).
Piesman, J. Experimental acquisition of the Lyme disease spirochete, Borrelia burgdorferi, by larval Ixodes dammini (Acari, Ixodidae) during partial blood meals. J. Med. Entomol. 28, 259–262 (1991).
Pérez, D., Kneubühler, Y., Rais, O., Jouda, F. & Gern, L. Borrelia afzelii ospC genotype diversity in Ixodes ricinus questing ticks and ticks from rodents in two Lyme borreliosis endemic areas: Contribution of co-feeding ticks. Ticks Tick Borne Dis. 2, 137–142 (2011).
Andersson, M., Scherman, K. & Raberg, L. Multiple-strain infections of Borrelia afzelii: a role for within-host interactions in the maintenance of antigenic diversity? Am. Nat. 181, 545–554 (2013).
Hellgren, O., Andersson, M. & Raberg, L. The genetic structure of Borrelia afzelii varies with geographic but not ecological sampling scale. J. Evol. Biol. 24, 159–167 (2011).
Strandh, M. & Raberg, L. Within-host competition between Borrelia afzelii ospC strains in wild hosts as revealed by massively parallel amplicon sequencing. Philos. T. Roy. Soc. B 370 (2015).
Marsot, M. et al. Which forest bird species are the main hosts of the tick, Ixodes ricinus, the vector of Borrelia burgdorferi sensu lato, during the breeding season? Int. J. Parasitol. 42, 781–788 (2012).
Norte, A. C. et al. Do ticks and Borrelia burgdorferi s.l. constitute a burden to birds? Parasitol. Res. 112, 1903–1912 (2013).
Heylen, D., Adriaensen, F., Dauwe, T., Eens, M. & Matthysen, E. Offspring quality and tick infestation load in brood rearing great tits Parus major . Oikos 118, 1499–1506 (2009).
Heylen, D. J. A. & Matthysen, E. Effect of tick parasitism on the health status of a passerine bird. Funct. Ecol. 22, 1099–1107 (2008).
Dubska, L., Literak, I., Kocianova, E., Taragelova, V. & Sychra, O. Differential role of passerine birds in distribution of Borrelia spirochetes, based on data from ticks collected from birds during the postbreeding migration period in Central Europe. Appl. Environ. Microbiol. 75, 596–602 (2009).
Heylen, D., Fonville, M., van Leeuwen, A. D. & Sprong, H. Co-infections and transmission dynamics in a tick-borne bacterium community exposed to songbirds. Environ. Microbiol. 18, 988–996 (2016).
Heylen, D., Tijsse, E., Fonville, M., Matthysen, E. & Sprong, H. Transmission dynamics of Borrelia burgdorferi s.l. in a bird tick community. Environ. Microbiol. 15, 663–673 (2013).
Drent, P. J., van Oers, K. & van Noordwijk, A. J. Realized heritability of personalities in the great tit (Parus major). P. Roy. Soc. B-Biol. Sci. 270, 45–51 (2003).
Jacquet, M., Durand, J., Rais, O. & Voordouw, M. J. Cross-reactive acquired immunity influences transmission success of the Lyme disease pathogen, Borrelia afzelii. Infect. Genet. Evol. 36, 131–140 (2015).
Sinsky, R. J. & Piesman, J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J. Clin. Microbiol. 27, 1723–1727 (1989).
Gooskens, J., Templeton, K. E., Claas, E. C. & van Dam, A. P. Evaluation of an internally controlled real-time PCR targeting the ospA gene for detection of Borrelia burgdorferi sensu lato DNA in cerebrospinal fluid. Clin. Microbiol. Infec. 12, 894–900 (2006).
Schwaiger, M., Peter, O. & Cassinotti, P. Routine diagnosis of Borrelia burgdorferi (sensu lato) infections using a real-time PCR assay. Clin. Microbiol. Infec. 7, 461–469 (2001).
Wang, I. N. et al. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 151, 15–30 (1999).
Hofmeester, T. R. et al. Few vertebrate species dominate the Borrelia burgdorferi s.l. life cycle. Environ. Res. Lett. 11 (2016).
Kjelland, V., Stuen, S., Skarpaas, T. & Slettan, A. Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from migratory birds in Southern Norway. Acta Vet. Scand. 52 (2010).
Acknowledgements
Thanks to Tom Fluri, Gaétan Pheulpin, Anouk Sarr, and Olivier Rais for helping to create the B. afzelii-infected I. ricinus nymphs. Thanks to Claire Cayol for providing us with B. afzelii isolate Fin-Jyv-A3. This research was supported by the Fund for Scientific Research - Flanders Belgium (FWO) (grant G0.049.10) and the University of Antwerp (KP BOF UA 2015). Dieter Heylen is a postdoctoral fellow at the FWO. This work was also supported by a Swiss National Science Foundation grant to Maarten J. Voordouw (FN 31003A_141153). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
D.H., M.J.V. and H.S. conceived and designed the study. D.H., A.K. and N.V. performed the experiments. D.G. and A.G.-C. created the B. afzelii-infected nymphal ticks. M.J.V., H.S., K.V. and D.H. provided funding. D.H., H.S., and M.J.V. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Heylen, D., Sprong, H., Krawczyk, A. et al. Inefficient co-feeding transmission of Borrelia afzelii in two common European songbirds. Sci Rep 7, 39596 (2017). https://doi.org/10.1038/srep39596
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39596
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.