Abstract
We report the first occurrence of an icosahedral quasicrystal with composition Al62.0(8)Cu31.2(8)Fe6.8(4), outside the measured equilibrium stability field at standard pressure of the previously reported Al-Cu-Fe quasicrystal (AlxCuyFez, with x between 61 and 64, y between 24 and 26, z between 12 and 13%). The new icosahedral mineral formed naturally and was discovered in the Khatyrka meteorite, a recently described CV3 carbonaceous chondrite that experienced shock metamorphism, local melting (with conditions exceeding 5 GPa and 1,200 °C in some locations), and rapid cooling, all of which likely resulted from impact-induced shock in space. This is the first example of a quasicrystal composition discovered in nature prior to being synthesized in the laboratory. The new composition was found in a grain that has a separate metal assemblage containing icosahedrite (Al63Cu24Fe13), currently the only other known naturally occurring mineral with icosahedral symmetry (though the latter composition had already been observed in the laboratory prior to its discovery in nature). The chemistry of both the icosahedral phases was characterized by electron microprobe, and the rotational symmetry was confirmed by means of electron backscatter diffraction.
Similar content being viewed by others
Introduction
Quasicrystals1,2, short for quasiperiodic crystals, are solids able to violate the conventional rules of crystallography because their structure is “quasiperiodic” rather than periodic; that is, their atomic density can be described by a finite sum of periodic functions with periods whose ratio is irrational. Their diffraction pattern consists of true Bragg peaks whose positions can be expressed as integer linear combinations of D integer linearly independent wavevectors where D is greater than the number of space dimensions. Among the quasicrystals made in the laboratory, many exhibit a crystallographically forbidden, three-dimensional icosahedral symmetry defined by D = 6 integer linearly independent wavevectors.
The first quasicrystalline phase found in nature, icosahedrite Al63Cu24Fe133,4, displayed a five-fold symmetry in two dimensions and icosahedral symmetry in three dimensions and was found in the Khatyrka meteorite, a CV3 carbonaceous chondrite5,6,7. The discovery represented a breakthrough in mineralogy and in condensed matter physics. Then, a second quasicrystal, decagonite Al71Ni24Fe58,9, was found in the same meteorite, and it was the first mineral to exhibit the crystallographically forbidden decagonal symmetry. Both icosahedrite and decagonite, however, showed compositions matching those of synthetic quasicrystalline phases found earlier10,11 in the laboratory at standard pressure.
Here we report the first icosahedral quasicrystal discovered in nature prior to being synthesized in the laboratory. It belongs to the Al-Cu-Fe system and exhibits the composition Al62.0(8)Cu31.2(8)Fe6.8(4), which is outside the measured equilibrium stability field at standard pressure of the previously reported Al-Cu-Fe quasicrystal12,13,14 (AlxCuyFez, with x between 61 and 64, y between 24 and 26, z between 12 and 13%). The new icosahedral phase was found in one of the meteoritic fragments of the same Khatyrka meteorite recovered from an expedition to the Koryak Mountains in far eastern Russia in 20115,7 as a result of a search for material that would provide information on the origin of icosahedrite, the first natural quasicrystal. The fragment is labeled Grain 126A to distinguish it from others of Grain 126. All recovered fragments of Khatyrka including Grain 126 have been shown to have CV3-like oxygen isotopic compositions6,15,16, confirming their common meteoritic origin.
Most of the Khatyrka meteoritic fragments display evidence of an impact shock that generated a heterogeneous distribution of pressures and temperatures in which some portions of the meteorite exceeded 5 GPa and 1200 °C15. Other fragments of Grain 126 have previously led to the discovery of novel phases, including the new polymorph of Al, steinhardtite17, as well as other new crystalline Al-Cu-Fe alloys18. Other phases found include ringwoodite, coesite, stishovite, magnetite, diopside, forsterite, clinoenstatite, sodalite, nepheline, pentlandite, Cu-bearing troilite, icosahedrite, khatyrkite (CuAl2), cupalite (CuAl), taenite, Al-bearing trevorite, and Al-bearing taenite13.
Results
Description of the sample
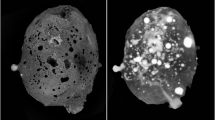
Grain 126A is dark grey in reflection under white incident light with visible shinier fragments reflecting the metallic constituents. Its small size – around ~0.4 mm in diameter –precludes determination of detailed optical properties of the minerals. A backscattered electron (BSE) image of 126A is shown in Fig. 1A. The two primary constituents of Grain 126A are: (1) large, disconnected Al-Cu-Fe metal assemblages (lighter, Fig. 1A); and, intimately surrounding these metal assemblages, (2) matrix material consisting of silicate glass and crystals of olivine and spinel (darker, Fig. 1A). Averaged and representative analyses of the different metal phases are presented in Table 1.
Backscattered electron images of Grain 126A.
(A) Grain 126A; red dashed box indicates the region to be enlarged in (B). (B) The area where there are the three metal assemblages containing the two different icosahedral phases; red dashed boxes (indicated as 1, 2 and 3) indicate the regions to be enlarged in panels on right. Panels 1, 2 and 3 show the different associations of minerals in the three metal assemblages.
Analysis of selected Al-Cu-Fe fragments
Al-Cu-Fe metals occur as small grains (Fig. 1B), generally irregular in shape and never spherical. They have a cuspate-lobate morphology, with cusps tending to point into the metal grains. The large Al-Cu-Fe metal fragments mostly comprise khatyrkite (CuAl2, with up to 2.68 elemental weight % Fe) and variable amounts of an unnamed (Al,Cu)Fe phase18 corresponding to the β phase in the Al-Cu-Fe system14,19. The β phase in 126A has the compositional range Al55–60Cu38–43Fe1–3, which overlaps with that of a known synthetic analogue. In addition to the β phase, the metal regions contain other previously unobserved Al-Cu-Fe phases. One is an unnamed phase with composition Al73Fe19Cu8, ideally Al3(Fe,Cu)18, corresponding to the λ phase in the Al-Cu-Fe system19.
Immediately adjacent to the λ grain are icosahedrite grains (denoted ‘i-phase I’). There are other metal grains that bear the same icosahedral quasicrystalline symmetry as icosahedrite but have a composition Al62.0(8)Cu31.2(8)Fe6.8(4) (denoted ‘i-phase II’), which is significantly outside the measured equilibrium stability field of icosahedrite12,13,14 (AlxCuyFez, with x between 61 and 64, y between 24 and 26, z between 12 and 13%), at standard pressure, even at temperatures up to 740 °C. Like β and λ, i-phase II is a previously unobserved phase. The i-phase II grains are completely enveloped by the β phase. The metal assemblages containing the i-phase II grains also have khatyrkite and metallic Al with a composition Al97–98Cu2–3.
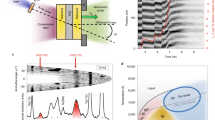
Both i-phase I and i-phase II were studied by means of electron backscattered diffraction (EBSD). The EBSD patterns obtained (Fig. 2) display a pentagonal symmetry in their Kikuchi patterns. The sharpness of the Kikuchi bands indicates the quasicrystals have a well-ordered structure with icosahedral symmetry, containing at most minor structural defects. As expected, the EBSD software, which is designed to index crystalline phases, was unable to index this pattern in any standard crystal system.
Electron backscatter diffraction patterns of the two icosahedral phases (the three patterns in the first row are from i-phase I, whereas those in the second row are from i-phase II) obtained from the regions labeled as i-phases in Fig. 1.
The patterns match those predicted for a face-centered icosahedral quasicrystal.
Discussion
The discovery of an icosahedral Al-Cu-Fe quasicrystal with a composition far from that of any known ideal, stable quasicrystal is notable for several reasons. It is only the third example of a natural quasicrystal to be found anywhere, all from fragments of the same Khatyrka meteorite; and it is the first documented example of the coexistence of two different Al-Cu-Fe i-phase compositions. Furthermore, it is the first example of a composition discovered in nature prior to being discovered in the laboratory.
The Al-Cu-Fe ternary phase diagram at standard pressure has been systematically studied around the icosahedral region12,13,14. Icosahedrite, Al63Cu24Fe13 or equivalently i-phase I, lies within the stability field for the icosahedral quasicrystal phase as measured at all temperatures below melting. By contrast, i-phase II, Al62.0(8)Cu31.2(8)Fe6.8(4), has a chemical composition that lies significantly outside the stability field at standard pressure for all temperatures below melting, for example, outside the stability field at room temperature first reported by Bancel12 (dashed area in Fig. 3) as well as at elevated temperatures up to 740 °C. A composition closer to that of i-phase II described here was reported by Zhang et al.14,19 during investigations on the Al-Cu-Fe system with low Fe content starting from an alloy with composition Al56.8Cu37Fe6.2 and annealed at 660 °C. Based on scanning electron microscopy (SEM) and X-ray powder diffraction measurements (which are not as precise as the methods reported here), they claimed an icosahedral phase with composition Al62.3Cu28.6Fe9.1, with significantly higher percentage of Fe and lower Cu than observed here in i-phase II. Gratias et al.20 found that at 680 °C, the stability field of the i-phase extends approximately over a triangle with vertices (62.4, 24.4, 13.2), (65, 23, 12) and (61, 28.4, 10.6), in terms of (Al, Cu, Fe) atomic %. They reported that this region splits schematically into 3 fields: (i) the perfect icosahedral phase, which is stable down to the lowest possible annealing temperature where atomic diffusion is active in a tiny region of composition close to Al62.3Cu24.9Fe12.8; (ii) a well-defined periodic phase with rhombohedral structure, which transforms reversibly into the icosahedral phase near 710 °C in the lower part of the triangle; (iii) a complex region characterized by additional diffraction effects (peak broadening, line-shapes, etc.), which may correspond to various approximant structures closely related to the i-phase. However, none of these earlier studies found evidence of the i-phase II composition.
Subsolidus projection of the ternary Al-Cu-Fe phase diagram in the vicinity of the icosahedral phase (modified after Bancel 12).
Shaded regions indicate pure phase fields and tie lines bound two-phase regions. The maximal extent of the icosahedral phase occurs within the boxed area labelled i-region. The dark shading shows the section of the i-region in which transformations have been identified. Empty red and light blue spheres correspond to data from i-phase I (icosahedrite) and i-phase II, respectively. Errors within the size of the symbols.
One possible explanation for why the i-phase II has not been found in earlier studies is that i-phase II is a kinetically stabilized composition, only preserved because of very rapid quench, and is thermodynamically unstable at any pressure and temperature. Previous investigations of Khatyrka samples15 have provided ample evidence that the meteorite experienced an impact-induced shock that generated a highly heterogeneous distribution of pressures and temperatures with conditions as high as 5 GPa and 1,200 °C, followed by rapid cooling. On this basis, the kinetic stabilization explanation is plausible. On the other hand, it is notable that fragments of 126A containing i-phase I, as illustrated in panel 1 in Fig. 1, have textures qualitatively similar to fragments with i-phase II, as shown in panels 2 and 3. The metallic phases observed with i-phase I appear to have formed according to a predictable solidification sequence along a liquid line of descent, largely consistent with the equilibrium phase diagram for Al-Cu-Fe, beginning with the formation of the λ phase and including the formation of an icosahedral phase with a composition in the equilibrium stability field at standard pressure. Based on their texture, the fragments containing i-phase II appear to have cooled at a similar rate but starting from a different initial composition, such that the solidification process began with i-phase II and skipped the λ phase. In that case, i-phase II could have a composition in a stability field for i-phase that has shifted or expanded due to the high P-T induced by the shock, and likely be a new high-pressure phase, crystallized from shock-induced melt under high pressure. This latter possibility could be explored in the laboratory by beginning with a liquid with the composition of i-phase II at high P-T and checking if the i-phase II solid forms when high pressure is maintained as the temperature decreases. Combining studies of natural quasicrystals, high-pressure diamond anvil experiments21,22, and laboratory shock experiments23, we plan to test this hypothesis and develop a better understanding of the kinetic and thermodynamic stability of quasicrystals.
Methods
Sample characterization techniques
The sample studied here (Grain 126A) was investigated by means of SEM (scanning electron microscopy), EBSD (electron backscatter diffraction), and EMP-WDS (electron microprobe, wavelength dispersive spectrometry) techniques.
Scanning Electron Microscopy
The sample was impregnated with epoxy and mechanically polished in flowing water with Al2O3-abrasive papers down to 5 μm and loose diamond powder down to 0.25 μm, followed by three hours of vibrational polishing in colloidal silica (30 nm in dia.) solution. Carbon coat was applied for SEM, EDS and EMP analyses, then removed for EBSD studies. The sample was examined on the Caltech GPS ZEISS 1550VP field emission SEM equipped with an angle-sensitive back-scattered electron detector, a 80 mm2 active area Oxford X-Max Si-drift-detector EDS, and an HKL EBSD system. Imaging, mapping, semi-quantitative EDS analysis, and EBSD of the previously identified regions were conducted using the SmartSEM, the AZtec and the Channel 5 softwares. Analyses used 20 kV accelerating potential and a 120 μm field aperture in high current mode (~6 nA probe current) for EBSD analysis and 15 kV for EDS analysis.
Electron microprobe
After location of the i-phases and identification of major and minor elements by SEM, three regions were re-analyzed for Al, Cu, Fe, Cr, Ni, Si, Mg and Ca using WDS on a five-spectrometer JEOL 8200 electron microprobe in the GPS analytical facility at Caltech. High spatial resolution was achieved using conditions of 12 kV, 5 nA, and a focused beam. Pure metal standards were used. Counting times were 20 s on-peak and 10 s each on high and low background positions. Data reduction used the CITZAF routine built into the Probe for EPMA software.
Additional Information
How to cite this article: Bindi, L. et al. Collisions in outer space produced an icosahedral phase in the Khatyrka meteorite never observed previously in the laboratory. Sci. Rep. 6, 38117; doi: 10.1038/srep38117 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Levine, D. & Steinhardt, P. J. Quasicrystals: A new class of ordered structures. Phys. Rev. Lett. 53, 2477–2480 (1984).
Shechtman, D., Blech, I., Gratias, D. & Cahn, J. Metallic phase with long-range orientational order and no translational symmetry. Phys. Rev. Lett. 53, 1951–1953 (1984).
Bindi, L., Steinhardt, P. J., Yao, N. & Lu, P. J. Natural Quasicrystals. Science 324, 1306–1309 (2009).
Bindi, L., Steinhardt, P. J., Yao, N. & Lu, P. J. Icosahedrite, Al63Cu24Fe13, the first natural quasicrystal. Am. Mineral. 96, 928–931 (2011).
Steinhardt, P. J. & Bindi, L. In search of natural quasicrystals. Rep. Progr. Phys. 75, 092601–092611 (2012).
MacPherson, G. J. et al. Khatyrka, a new CV3 find from the Koryak Mountains, Eastern Russia. Met. Plan. Sci. 48, 1499–1514 (2013).
Bindi, L. & Steinhardt, P. J. The quest for forbidden crystals. Mineral. Mag. 78, 467–482 (2014).
Bindi, L. et al. Natural quasicrystal with decagonal symmetry. Sci. Rep. 5, 9111 (2015).
Bindi, L. et al. Decagonite, Al71Ni24Fe5, a quasicrystal with decagonal symmetry from the Khatyrka CV3 carbonaceous chondrite. Am. Mineral. 100, 2340–2343 (2015).
Tsai, A. P., Inoue, A. & Masumoto, T. A. A stable quasicrystal in Al-Cu-Fe system. Jap. J. Appl. Phys. 26, L1505 (1987).
Tsai, A. P., Inoue, A. & Masumoto, T. A. New decagonal Al-Ni-Fe and Al-Ni-Co alloys prepared by liquid quenching. Mater. Trans. JIM 30, 150–154 (1989).
Bancel, P. A. Order and disorder in icosahedral alloys. Quasicrystals. In: Series on Directions in Condensed Matter Physics, World Scientific, Edited by DiVincenzo, D. P. & Steinhardt, P. J. 16, 17–55 (1991).
Gayle, F. W. et al. The Al-Cu-Fe Phase Diagram: 0 to 25 At. pct. Fe and 50 to 75 at. pct. Al – equilibria involving the icosahedral phase; Met. Trans 23A, 2409–2417 (1992).
Zhang, L., Schneider, J. & Lück, R. Phase transformations and phase stability of the AlCuFe alloys with low-Fe content. Intermetallics 13, 1195–1206 (2005).
Hollister, L. S. et al. Impact-induced shock and the formation of natural quasicrystals in the early solar system. Nat. Comms. 5, 3040, doi: 10.1038/ncomms5040 (2014).
Bindi, L. et al. Evidence for the extra-terrestrial origin of a natural quasicrystal. Proc. Natl Acad. Sci. USA 109, 1396–1401 (2012).
Bindi, L. et al. Steinhardtite, a new body-centered-cubic allotropic form of aluminum from the Khatyrka CV3 carbonaceous chondrite. Am. Mineral. 99, 2433–2436 (2014).
Ma, C., Lin, C., Bindi, L. & Steinhardt, P. J. Discovery of new Al-Cu-Fe minerals in the Khatyrka CV3 meteorite. 79thAnn. Meeting Met. Soc., PDF no. 6017 (2016).
Zhang, L. & Lück, R. Phase diagram of the Al–Cu–Fe quasicrystal-forming alloy system: I. Liquidus surface and phase equilibria with liquid. Zeit. Metallk. 94, 91–97 (2003).
Gratias, D. et al. The phase diagram and structures of the ternary AlCuFe system in the vicinity of the icosahedral region. J. Non-Cryst. Solids 153, 482–488 (1993).
Stagno, V. et al. Icosahedral AlCuFe quasicrystal at high pressure and temperature and its implications for the stability of icosahedrite. Sci. Rep. 4, 5869 (2014).
Stagno, V. et al. Quasicrystals at extreme conditions: The role of pressure in stabilizing icosahedral Al63Cu24Fe13 at high temperature. Am. Mineral. 100, 2412–2418 (2015).
Asimow, P. D. et al. Shock synthesis of quasicrystals with implications for their origin in asteroid collisions. Proc. Nat. Acad. Sci. USA 113, 7077–7081 (2016).
Acknowledgements
We wish to thank the 2011 expeditionary team composed by: Christopher L. Andronicos, Vadim V. Distler, Michael P. Eddy, Alexander Kostin, Valery Kryachko, Glenn MacPherson, William Steinhardt and Marina Yudovskaya. Special thanks are also due to Lincoln Hollister and Nan Yao for their invaluable help in the different stages of this project. This work was supported in part by “Progetto di Ateneo 2015” of the University of Florence, Italy (L.B.) and the National Science Foundation-MRSEC program through New York University (DMR-0820341; P.J.S.). SEM, EBSD and EPMA analyses were carried out at the Caltech GPS Division Analytical Facility, which is supported, in part, by NSF Grants EAR-0318518 and DMR-0080065. We are also grateful to the anonymous donor who supported the 2011 expedition to Chukotka through a grant to Princeton University (P.J.S., Principal Investigator).
Author information
Authors and Affiliations
Contributions
The study was conceived and guided by L.B. and P.J.S., who also led the research team. C.L. and C.M. performed the SEM studies. C.M. found i-phase II and carried out the electron backscattered diffraction and electron microprobe studies. L.B. and P.J.S. wrote the paper. All the authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Bindi, L., Lin, C., Ma, C. et al. Collisions in outer space produced an icosahedral phase in the Khatyrka meteorite never observed previously in the laboratory. Sci Rep 6, 38117 (2016). https://doi.org/10.1038/srep38117
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep38117
This article is cited by
-
A naturally occurring Al-Cu-Fe-Si quasicrystal in a micrometeorite from southern Italy
Communications Earth & Environment (2024)
-
Quasicrystals at high pressures and temperatures: a review
Rendiconti Lincei. Scienze Fisiche e Naturali (2023)
-
Beauty of Order and Symmetry in Minerals: Bridging Ancient Greek Philosophy with Modern Science
Foundations of Science (2023)
-
Quasicrystals: A New Class of Structurally Complex Intermetallics
Journal of the Indian Institute of Science (2022)
-
Photonic, Low-Friction and Antimicrobial Applications for an Ancient Icosahedral/Quasicrystalline Nano-composite Bronze Alloy
Transactions of the Indian Institute of Metals (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.