Abstract
Based on the galactinol synthase (AnGolS1) fragment sequence from a cold-induced Suppression Subtractive Hybridization (SSH) library derived from Ammopiptanthus nanus (A. nanus) seedlings, AnGolS1 mRNA (including the 5′ UTR and 3′ UTR) (GenBank accession number: GU942748) was isolated and characterized by rapid amplification of cDNA ends polymerase chain reaction (RACE–PCR). A substrate reaction test revealed that AnGolS1 possessed galactinol synthase activity in vitro and could potentially be an early-responsive gene. Furthermore, quantitative real-time PCR (qRT-PCR) indicated that AnGolS1 was responded to cold, salts and drought stresses, however, significantly up-regulated in all origans by low temperatures, especially in plant stems. In addition, the hybridization signals in the fascicular cambium were strongest in all cells under low temperature. Thus, we propose that AnGolS1 plays critical roles in A. nanus low-temperature stress resistance and that fascicular cambium cells could be involved in AnGolS1 mRNA transcription, galactinol transportation and coordination under low-temperature stress.
Similar content being viewed by others
Introduction
Environmental stresses are the most influence factors affecting plant survival and distribution worldwide1. Abiotic stresses, such as low temperature, drought, and salt stresses, can seriously affect the normal growth, development, and yield of plants2,3,4,5. To reduce the potential damage from these stresses, plants have evolved complex strategies, such as the accumulation of certain small soluble molecules (such as proline, mannitol, and oligosaccharide) to increase the osmotic pressure in cells, thereby improving plant stress resistance6,7. Raffinose family oligosaccharides (RFOs) are the second largest group of sugars in plant tissues and are nonstructural carbohydrates that play essential roles in plant growth and development8. In addition to enhancing abiotic stress tolerance, RFOs are an alternative to sucrose and starch for glucide transport and storage9,10.
In the galactinol-dependent pathway, RFOs are synthesized from sucrose and galactose precursors that are joined by an (α-1, 6) glycosidic bond. However, in the galactinol-independent pathway, two RFOs interact to form higher-order RFOs members11. Galactinol is synthesized from UDP-D-galactose and myo-inositol by galactinol synthase (GolS; EC 2.4.1.123), which catalyzes the first committed step in the RFOs biosynthetic pathway in plants12,13,14. Thus, GolS is a key regulator in RFOs synthesis and a restriction enzyme for RFOs accumulation9,15,16. Previous studies have shown that GolS was important in the physiology of plant stress resistance, photosynthate translocation and seed physiology. A. thaliana AtGolS1 and AtGolS2 were up-regulated by drought and salt stresses, whereas AtGolS3 was up-regulated by cold stress6. Coffea arabica L. greatly accumulated GolS under drought and salt stresses17. Pisum sativum L. PsGolS increased in roots and epicotyls of seedlings after dehydration, and its level in epicotyls was obviously higher than in roots during dehydration18. AtGolS2 is involved in processes related to seed longevity, but AtGolS1 does not exert similar action19. Some GolS genes are involved in basic metabolic activities, such as carbon storage and the seasonal mobilization of carbohydrates20,21,22. After seed development, seeds accumulate an abundance of RFOs, and GolS activity gradually increases before stachyose rises, reaching its peak after flowering and decreasing to a plateau in dry seeds13,23,24,25. Fleshy fruits such as melons actively synthesize raffinose and stachyose, but the contents of both sugars are very low, thereby suggesting that RFOs are rapidly catabolized26,27. During heat stress, although the VvGolS1 expression in Vitis vinifera increased, galactinol derivatives were not detected upon heat stress, and raffinose and stachyose were merely generated after heat stress28. Additionally, plant growth was impaired in GolS-silenced transgenic plants20, and GolS activities were related to different isozymes with varying GolS functions6,9,17.
The genus Ammopiptanthus (Leguminosae) comprises only two relict species: A. mongolicus and A. nanus endemically distributed in the South Gobi desert and the desert in Central Asia, respectively. A. nanus is distributed only in a narrow altitudinal strip between 1,800 and 2,800 m in the Kezilesu Kirgiz Autonomous Prefecture of Xinjiang and the Soviet Union. In these regions, the temperature varies dramatically from −30 °C to 47.6 °C; at the same time, the climate is extremely arid because annual precipitation is lower than 20 mm, whereas annual evaporation is higher than 2,000 mm29,30.
To understand the mechanism of abiotic stress tolerance at the molecular level, we acquired a GolS homologous sequence from a cold-induced SSH library derived from A. nanus seedlings. The gene was named AnGolS1, and its full-length cDNA was cloned. In this paper, it was demonstrated that AnGolS1 possesses the ability to synthesize galactinol in vitro and might be an early-responsive gene based on the results of a substrate reaction test. QRT-PCR was used to analyze gene transcript accumulation under different stresses, and the results indicated that AnGolS1 expression can be responded to cold, salts and drought stresses, significantly up-regulated by low-temperature stress. In addition, in situ hybridization (ISH) was utilized to explore AnGolS1 expression levels in the cells of different tissues under low-temperature stress. Such efforts will help us better understand the mechanism of abiotic stress tolerance, so as to develop and utilize A.nanus and to enhance crops toleration.
Results
Cloning and Characterization of the Full-length AnGolS1 cDNA
An AnGolS1 fragment was obtained from a cold-induced SSH library derived from A. nanus seedlings, and then the 5′-end and 3′-end sequences were amplified by 5′- and 3′-RACE, respectively. The full-length cDNA of AnGolS1 (GenBank Accession No.: GU942748), which had a length of 1,244 bp, was spliced and flanked by stretches of 98 and 159 bp at the 5′- and 3′-untranslated regions, respectively. The cDNA encoded a polypeptide of 328 amino acids with a predicted molecular weight of approximately 37.576 kD.
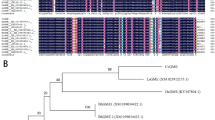
AnGolS1 shares a high degree of homology with a variety of GolS proteins from A. mongolicus (AmGolS, 98%), Glycine max (GmGolS1, 85.80%), Medicago truncatula (MtGolS, 82.32%), Nicotiana tabacum (NtGolS2, 76.35%), and A. thaliana (AtGolS1, 73.26%) (Fig. 1). These homologous proteins, ranged between 328 and 344 amino acids, have seven gaps, six in the region of residues 3 to 35 in the N- and C-termini. Thus, high diversity was found in the first and last 35 positions of the N-termini and C-termini, respectively. In addition, AnGolS1 includes a substrate binding site and a manganese binding site (Fig. 1), a Pfam domain from amino acids 28 to 272, and a region of low compositional complexity between amino acids 311 and 320.
Alignment of the AnGolS1 amino acid sequence with AmGolS (ABF66656.1), GmGolS2 (XP_003521658.1), MtGolS (KEH24464.1), NtGolS2 (AHM22934.1), and AtGolS1 (NP_182240.1).
The starting and ending sites of the conserved domain are marked with a one-headed arrow. The substrate binding site is marked with a double-headed arrow. The manganese binding site is marked with a triangle.
Phylogenetic Analysis of AnGolS1
A phylogenetic tree was constructed to determine the evolutionary relationships among AnGolS1 and other plant GolS proteins based on the alignment of the GolS proteins (Fig. 2). The available GolS proteins could be classified into three major clades (I, II, and III). AnGolS1 grouped with AmGolS, MtGolS, MsGolS, MfGolS, GmGolS2, and VhGolS in clade III. These species in clade III belong to Leguminosae, nevertheless, clades I and II belong to Solanaceae and Brassicaceae, respectively.
Phylogenetic tree of AnGolS1 and other plant GolS sequences.
Evolutionary relationships were inferred using the neighbor-joining method. The bootstrap consensus tree inferred from 1,000 replicates is taken to represent the evolutionary history of the taxa analyzed. The bars represent evolutionary distance. The GolS proteins used in the phylogenetic tree analysis are from the plants Ammopiptanthus nanus (AnGolS1, ADF43063.1), Ammopiptanthus mongolicus (AmGolS, ABF66656.1), Glycine max (GmGolS2, XP_003521658.1), Medicago truncatula (MtGolS, KEH24464.1), Medicago sativa (MsGolS, AAM97493.1), Medicago falcata (MfGolS, ACM50915.1), Vicia hirsuta (VhGolS, AGW51291.1), Nicotiana tabacum (NtGolS2, AHM22934.1), Solanum tuberosum (StGolS2-like, XP_006340652.1), Arabis alpina (AaGolS, KFK37461.1), Solanum tuberosum (StGolS, ADW78849.1), Solanum lycopersicum (SlGolS2, NP_001234668.1), Brassica napus (BnGolS, CDX80084.1), Solanum commersonii (ScGolS, ADW78842.1), Capsicum annuum (CaGolS, ABQ44212.1), Arabidopsis thaliana (AtGolS1, NP_182240.1), and Capsella rubella (CrGolS, XP_006302453.1).
Induced expression and functional characterization of AnGolS1 in E. coli
A specific 43-kD fusion protein was expressed in BL21 cells which transformed with pET-30a-AnGolS1 and were induced with isopropyl β-D-1-thiogalactopyranoside (IPTG). The results suggested that the best time point for induction was 5 h, at which time the expressed fusion protein was at maximum levels (Fig. 3A). Thus, we chose to extract fusion proteins from 5 h induced cells (100 mL).
The induction, extraction and analysis of fusion protein enzyme activity.
(A) Extraction of the induced total proteins. M, protein marker. Control, empty vector (pET-30a+) cells were treated with IPTG 6 h. Label 0, pET-30a-AnGolS1 cells cultured for 6 h under normal conditions. Labels 1 to 6, pET-30a-AnGolS1 cells treated with IPTG for 1 to 6 h. (B) Detection of the purified fusion protein as a band of approximately 43 kD, between 40 kD to 45 kD. (C) Changes in the galactinol curve during the substrate reaction test. The horizontal and longitudinal coordinates represent the reaction time and the generation of galactinol, respectively.
The extracted fusion proteins were used to purify His-fusion proteins, and then the His-fusion proteins were recovered and concentrated. The concentrated proteins had a single vivid 43-kD band but did not exhibit other bands (Fig. 3B). The recovered fusion protein was used in an enzyme activity assay and we found that the reaction product galactinol was rapidly produced after reacting for 5 min at 30 °C, continuously increased at a steady rate for 40 min and then remained at a fairly constant level from 40 min to 60 min (Fig. 3C). These results demonstrated that the fusion protein possessed galactinol synthase activity in vitro, and its enzyme activity was very robust.
Expression analysis of AnGolS1 in A. nanus
qRT-PCR was performed to analyze AnGolS1 transcript accumulation in A. nanus seedlings in different organs under different stresses. Under 4 °C treatment, AnGolS1 expression in stems and leaves continually increased from 0 h to 24 h and then reduced from 24 h to 48 h, increased respectively 334.5- and 13.4-fold at 24 h compared with controls (Fig. 4A,C), however, that in roots continuously increased throughout the entire duration of treatment and raised 103.6-fold at 48 h (Fig. 4B). Under 20% PEG8000 treatment, the gene expression in stems increased at 4 h and that in leaves significantly decreased 0.13-fold at 6 h compared with controls, but that in roots did not notablely change throughout the entire duration of treatment (Fig. 4D,F). Under 0.25 M NaCl treatment, AnGolS1 expression increased in roots and stems at 24 h, reduced in stems at 12 h compared with controls (Fig. 4H,I), however, that in leaves did not evidently change (Fig. 4G). Thus, it was suggested that AnGolS1 was responded to cold, salt and drought stresses, up-regulated by low temperature in all tissues and played a less prominent part in salt and drought stresses compared with low-temperature stress.
AnGolS1 expression profiles under different stress treatments.
Relative expression in roots, stems and leaves under 4 °C low-temperature treatment (A–C), under 20% PEG8000 treatment (D–F) and under 0.25 M NaCl treatment (G–I). The data were presented as the mean ± SE of three independent experiments. Different letters above the columns indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) according to Duncan’s multiple range test.
Tissue distribution of AnGolS1
In situ hybridization was performed on different tissues of A. nanus. Two probes (named GST7, sense, and GSSP6, antisense) were used for hybridization (Table S3). The hybridization signal of the antisense probe GSSP6 was intense in the cold treatment tissues and lower in the normal tissues (Fig. 5C,D,I,J,O,P). At low temperature, the signal appeared near the primary phloem and was more noticeable compared with normal roots (Fig. 5E,F). Moreover, a hybridization signal in stems was clearly observed in the fascicular cambium between the phloem and xylem and was many times stronger than in normal stems (Fig. 5K,L). A stronger hybridization signal was observed in fascicular cambium hypocotyls at low temperature (Fig. 6). In addition, a strong hybridization signal still appeared in leaves under low temperature (Fig. 5Q,R). Meanwhile, the negative-control hybridizations with the sense-strand probe GST7 did not result in signal detection in all tissues (Fig. 5A,B,G,H,M,M). These results were consistent with the AnGolS1 transcript expression results and further confirmed that cold stress significantly regulated AnGolS1 accumulation.
In situ hybridization with AnGolS1 sense and antisense probes of A. nanus seedling roots, stems and leaves tissues.
Roots, stems and leaves were hybridized with sense probe GST7 (A,B,G,H,M,N) and antisense probe GSSP6 (C,D,I,J,O,P), respectively. Roots, stems and leaves were hybridized with antisense probe GSSP6 after 4 treatment for 24 °C hours (E,F,K,L,Q,R). The white arrows indicated the hybridization signal. PX, primary xylem; PPh, primary phloem; Fca, fascicular cambium; Co, cortex.
In situ hybridization with AnGolS1 sense and antisense probes of A. nanus seedling hypocotyls.
The crosscutting hypocotyls were hybridized with sense probe GST7 (A,B) and antisense probe GSSP6 after cold treatment (C–E), the slitting hypocotyls were hybridized with GST7 (F,G), and GSSP6 after cold treatment (H,I). The white arrows indicated the hybridization signal. VC, vascular bundle; SV, spiral vessel; Fca, fascicular cambium; PX, primary xylem.
Discussion
A. nanus has evolved a very strong ability for cold tolerance and is suitable to study the cryoprotectant mechanisms of woody species. To understand the cryoprotectant mechanisms of A. nanus, the full-length AnGolS1 gene was cloned and its possible function was analyzed from various perspectives.
Different GolS enzymes exhibit different penta-peptide characteristics in their C termini, such as AnGolS1, AmGolS, NtGolS2 and AtGolS1 express the characteristics of APSAA, whereas MtGolS shows ASNAA characteristics (Fig. 1). In addition, XvGolS, ArGolS1 and BnGolS also emerge APSAA characteristics31, BhGolS1 demonstrates PPTA characteristics32 and ZmGolS37 only has a proline residue in its C terminus, but other ZmGolS genes demonstrate APSAA characteristics33. The differences in the termini are likely connected with GolS function. Furthermore, the AnGolS1 protein does not possess a signal peptide site, which suggested that this protein is not a secreted protein.
Galactinol, which is synthesized by GolS, is an essential substrate for the synthesis of RFOs. Thus, GolS activity limits RFO accumulation. In AtGolS1 mutant plants, galactinol and RFOs did not accumulate under heat-induced stress34, and the double mutants (AtGolS1 T-DNA insertion mutant on an AtGolS2 background) did not tolerate drought tolerance35. Previous studies demonstrated that RFOs possess a cryoprotectant role for photosystem II (PS II), and the level of galactinol in the A. thaliana raffinose synthase (RS14) mutant was higher than that in the wild-type plants during cold treatment36. The dehydration-responsive element binding proteins (DREB1A/CBF3) and C-repeat binding factor (CBF1) transgenic plants accumulated large amounts of galactinol and RFOs, and plant cold tolerance was obviously improved37,38,39,40. Fundamentally, the increased expression of AtGolS3 directly caused increased RFO levels in DREB1A transgenic plants37. In qRT-PCR experiments, we found that AnGolS1 was significantly up-regulated in all organs by low-temperature stress, and its expression in stems was higher 334.5-fold than that in controls (Fig. 4C). Thus, two assumptions were made to predict the possible function of AnGolS1 at the tissue level. First, galactinol was rapidly synthesized in stems by AnGolS1, and then the majority was utilized to synthesize RFOs by raffinose synthase under low-temperature stress. Second, only a small portion of synthesized galactinol was utilized to synthesize RFOs; most galactinol directly acted to protect plants independently as it was found that RFOs and galactinol could protect plant cells from oxidative damage under low temperature41. Besides, both the CsGolS1 overexpressing and galactinol-treated wild-type tobacco plants enhanced the resistance against pathogen infection and drought and high salinity stresses42. These results indicated that galactinol has a protecting function in biotic and abiotic stresses in plants. In addition, its expression in roots and leaves increased under low temperature, suggesting that this protection mechanism was also present in roots and leaves (Fig. 4A,B). Expression in roots and stems at 2 h increased under salt stress, but the varieties were smaller compared with the same tissues under low-temperature stress. Furthermore, similar variations were evident in stems at 48 h under PEG8000 stress.
In different plants, the GolS genes demonstrate differential tissue-specific expression. For example, CaGolS1 had relatively higher transcript abundance in coffee leaves than CaGolS2 and CaGolS3, but CaGolS2 was not present or low express in almost all tissues and CaGolS3 had higher abundance in flowers and roots than in leaves17. GhGolS1 in anthers was considerably higher than in leaves43. ZmGolS2 was regulated by drought stress, but ZmGolS3 was only present in mature seeds as a stored message44. Although the AnGolS1 protein demonstrates high similarity to AmGolS, MfGolS1, MsGolS, MtGolS, GmGolS2 and VhGolS, the functions of these proteins have large differences. MfGolS1 was up-regulated after cold treatment in senescent leaves, mature leaves and lateral roots but was not expressed in the petiole, stems and axial roots, and it was weakly induced by dehydration and salt stresses45. Moreover, MsGolS was also induced by cold stress45. Furthermore, the AmGolS expression began to increase at 12 h under a temperature of 4 °C, thus indicating that it is a late-responsive gene similar to AtGolS36, and it was regulated by drought, high salinity and ABA stresses46. However, although AnGolS1 can been regulated by PEG8000, salt and low-temperature, AnGolS1 is mainly up-regulated by low temperature, and its expression in stems at 6 h was increased 5.6-fold compared with controls. In addition, galactinol increased to 2 μg after reacting for 5 min during a substrate reaction test. Thus, we believe that AnGolS1 is likely to be an early-responsive gene. Besides, its expressions in leaves were depressed under salt or PEG8000 stresses.
It has been verified that Ajuga reptans plants contain two RFOs pools: one is an RFO storage pool in the mesophyll, and the other is an RFO transport pool in the companion cells of the phloem47. In warm- and cold-grown leaves of Ajuga reptans, the hybridization signals of ArGolS1 and ArGolS2 were observed in the mesophyll and phloem-associated intermediary cells, respectively, in ISH studies; thus, ArGols1 and ArGolS2 were speculated to associate with the synthesis of storage and transport RFOs, respectively48. In this paper, we observed that the hybridization signal in stems was very strongest in all tissues after low-temperature treatment and that the signal was mainly focused in the fascicular cambium surrounding the primary xylem. This appearance was observed in the fascicular cambium in crosscut hypocotyls and near the vascular bundle following slitting after low-temperature treatment. At the same time, we also found that hybridization signals clearly emerged in roots and leaves surrounding the primary xylem, but their signal strength was weaker than that in stems. Thus, we speculated that AnGolS1 is likely to be first synthesized in the fascicular cambium in stems and then synthesized galactinol was transported in proximity to other cells, such as the cortex, phloem and xylem, under low-temperature stress. More studies must be carried out to explore the function of the fascicular cambium cells in the cold tolerance of A. nanus and to determine how these cells regulate AnGolS1 gene expression.
In conclusion, we believe that AnGolS1 might aid A. nanus in resisting the harmful effects of low-temperature stress, and fascicular cambium cells could be involved in AnGolS1 mRNA transcription and then galactinol transportation and coordination under low-temperature stress. These hypotheses not only help us to better understand the important role that AnGolS1 plays in phloem loading, but will also further guide us to explore the metabolism regulatory mechanisms of low-temperature stress in A. nanus and enhance crop resistance via production practices employing transgenic technology.
Materials and Methods
Plant Materials
A. nanus seeds were collected from Wuqia County in Xinjiang Provence in China and cultivated in a sandy substrate with a relative humidity of approximately 60%. The seedlings with 6 to 8 leaves (grown for approximately 6–8 weeks) were subjected to 4 °C low-temperature, 0.25 M NaCl, or 20% PEG 8000 (instead of control watering) stresses, and each stress was, respectively assayed after 0, 6, 12, 18, 24 and 48 h of treatment. The roots, stems and leaves of these seedlings at different treatment times were gathered and utilized as samples for qRT-PCR. In addition, the roots, stems and leaves of seedlings at 24 h after low-temperature stress were utilized for in situ hybridization.
Primers
The primers used in this study are shown in Table S3.
RNA Extraction and cDNA Synthesis
Total RNA from roots, stems and leaves was individually extracted using the TaKaRa MiniBEST Plant Universal RNA Extraction Kit (Takara, Japan). First-strand cDNA was synthesized using Superscript III Reverse Transcriptase (Invitrogen, America).
5′-RACE and 3′-RACE
Based on the GolS segment sequence, gene-specific primers were designed and synthesized (Table S3). The 5′- and 3′-end sequences were obtained using the 5′ and 3′ RACE System for Rapid Amplification of cDNA Ends (Invitrogen). The inner 5′ and 3′ RACE PCR products were resolved on agarose gels, and the appropriate bands were excised and then purified and cloned into the pMD18-T vector for sequencing.
Full-length cDNA Amplification of AnGolS1
The full-length cDNA of AnGolS1 was deduced by sequence comparison and alignment with the 5′-RACE and 3′-RACE sequences using DNAMAN software. The AnGS F and AnGS R primers were designed based on the deduced cDNA to obtain the full-length sequence (Fig. S2A). The purified PCR product was cloned into the pMD18-T vector for sequencing as described above.
AnGolS1 fusion protein expression
AnGolS1 was expressed in Escherichia coli as a His-fusion protein using the plasmid vector pET-30a (+). The coding region of AnGolS1 was amplified with two primers (forward, AnGS F; reverse, AnGS R) that contained BamHI and HindIII restriction sites, respectively. After digestion with HindIII/BamHI, the fragment was inserted into pET-30a (+) using the same restriction sites and the recombinant vector was designated pET-30a-AnGolS1 (Fig. S2B). Empty pET-30a (+) and pET-30a-AnGolS1 were transformed into E. coli BL21 (DE3) cells.
E. coli cells transformed with pET-30a-AnGolS1 were treated with 1 mmol.L−1 IPTG and cultured for different amounts of time. Finally, 1 mL of treated cells were harvested, boiled and analyzed by Coomassie blue staining using 15% SDS–polyacrylamide gels.
Enzyme activity assay for fusion proteins
Total E. coli proteins were extracted from treated cells (100 mL) and purified with His-fusion proteins using Ni-NTA His-Bind Resin (Merck), and then enzyme activities were recovered using phosphate buffered saline (PBS). GolS activity was determined according to the method described by Peterbauer49. The final reaction volume (30 μL) for the GolS activity assay contained 10 μL of the recovered enzyme extract, 5 mM MnCl2, 5 mM UDP-galactose (Sigma), and 20 mM myo-inositol. To better assay the resulting quantity of galactinol, we designed a reaction time gradient, and after incubation at 30 °C, the reaction was terminated by adding 70 μL of 80% ethanol and boiling the mixture for 5 min. The extracted reaction products (10 μL) were analyzed by High Performance Liquid Chromatography (HPLC) with pulsed amperometric detection utilizing a Carbopac PA10 column (Dionex, Vienna)50. Control reactions contained PBS buffer instead of substrates (Fig. S3; Tables S1 and S2).
Bioinformatics Analyses
DNASTAR software was used to translate the open reading frame (ORF) and to calculate the molecular weight of the deduced protein. BLAST was performed on the NCBI server (http://www.ncbi.nlm.nih.gov/BLAST). The SignalP 4.1 Server was used to predict protein signal peptides (http://www.cbs.dtu.dk/services/SignalP/). Protein conserved domains were predicted on the NCBI server (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) and the SMART server (http://smart.embl-heidelberg.de/). Multiple sequence alignments and phylogenetic analysis were performed by using the ClustalW51 and GeneDoc52 programs. A phylogenetic tree was constructed using MEGA 4.053 from ClustalW alignments and the neighbor-joining method54 was used to construct a tree. Structural analysis of the deduced proteins was accomplished using SWISS-MODEL55,56 on the website (http://www.expasy.org), and WebLab ViewerLite 4.0 was used to display the 3D model (Fig. S1).
AnGolS1 expression in A. nanus
qRT-PCR was used to analyze AnGolS1 transcript accumulation in different tissues of A. nanus seedlings in a greenhouse under different stresses grown. Total RNA was isolated from leaves, stems, and roots by using the TaKaRa MiniBEST Universal RNA Extraction Kit (Takara, Japan), and first-strand cDNA was synthesized as mentioned above. This cDNA was used as a template in qRT-PCR with the primers AnGolS1 RT-F and AnGolS1 RT-R, which are specific to the AnGolS1 coding sequence. Actin-F and Actin-R primers were used to amplify the housekeeping gene Actin as an internal control. The qRT-PCR reactions were performed in triplicate for each sample using Real Master Mix (SYBR Green) (Tiangen, China) on a real-time PCR system (ABI 7500, USA) under the following conditions: 95 °C for 60 s, 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 68 °C for 60 s, 1 cycle of 95 °C for 15 s and 60 °C for 1 min, and 95 °C for 15 s. The specificity of each primer pair was validated by a dissociation curve. The experiment was repeated at least three times, and gene expression levels were calculated based on the method described by Pfaffl57. Significant differences in the data were analyzed according to Duncan’s multiple range test.
Synthesis and DIG-labeled probe
The primers GS-HF and GS-HR were used to synthesize the probe. The probe was labeled with digoxigenin-UTP via in vitro transcription with SP6 and T7 RNA polymerase following the manufacturer’s protocol (DIG RNA Labeling Kit (SP6/T7), Roche) (Fig. S4A,B).
Collection and fixation of A. nanus specimens
The roots, stems and leaves of seedlings at 24 h after low-temperature treatment were collected individually. After the specimens were fixed in a solution consisting of 2% paraformaldehyde, 2.5% glutaraldehyde, and 0.1 M NaH2PO4/Na2HPO4 (pH 7.4) at 4 °C overnight, dehydrated, cleared through an ethanol-to-xylene series and embedded in paraffin, as described by Pirttilä58.
In Situ Hybridization (ISH) protocol
The ISH protocol was modified from the recommended method of Abcam (http://www.abcam.com/protocols/ish-in-situ-hybridization-protocol). Paraffin-embedded tissue sections at 9 μm were dewaxed with xylene and rehydrated through a series of gradient-diluted ethanol solutions. Next, the slices were successively treated with proteinase K (Roche), acetic acid, gradient-diluted ethanol solutions and were air-dried. After pre-hybridization at 55 °C for 1 h, hybridization was carried out in a solution containing heat-denatured probe by incubating at 55 °C overnight. Post-hybridization was performed through a serially diluted saline-sodium citrate (SSC) gradient, followed by treatment with maleic acid buffer containing Tween 20. The slides were successively blocked with DNA blocking reagent (Roche) and detected with an anti-digoxigenin-AP Fab fragment (Roche) for 1 h at room temperature. The antibody-antigen complexes were subsequently visualized with NBT/BCIP (Roche) at a 1:50 dilution in alkaline buffer at 37 °C overnight. Finally, the slides were rinsed with distilled water for 5 min and air-dried.
Additional Information
How to cite this article: Liu, Y.D. et al. Molecular cloning and expression of an encoding galactinol synthase gene (AnGolS1) in seedling of Ammopiptanthus nanus. Sci. Rep. 6, 36113; doi: 10.1038/srep36113 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Osmond, C. et al. Stress physiology and the distribution of plants. Bioscience 37, 38–48 (1987).
Theocharis, A., Clément, C. & Barka, E. A. Physiological and molecular changes in plants grown at low temperatures. Planta 235, 1091–1105 (2012).
Sairam, R. & Tyagi, A. Physiology and molecular biology of salinity stress tolerance in plants. Current Science Bangalore 86, 407–421 (2004).
Shinozaki, K. & Yamaguchi-Shinozaki, K. Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Current Opinion in Plant Biology 3, 217–223 (2000).
Wang, W., Vinocur, B. & Altman, A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1–14 (2003).
Taji, T. et al. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant Journal 29, 417–426 (2002).
Levitt, J. Response of plants to environmental stresses. Water, radiation, salt and other stresses 2 (1980).
French, D. The Raffinose Family of Oligosaccharides. Advances in Carbohydrate Chemistry 9, 149–184 (1954).
Keller, F. & Pharr, D. M. Metabolism of carbohydrates in sinks and sources: galactosyl-sucrose oligosaccharides. Photoassimilate distribution in plants and crops: source-sink relationships. Marcel Dekker, New York, p. 157–183 (1996).
Kandler, O. & Hopf, H. Oligosaccharides based on sucrose (sucrosyl oligosaccharides), In Plant Carbohydrates I. Springer. p. 348–383 (1982).
Gangola, M. P. et al. Galactinol synthase enzyme activity influences raffinose family oligosaccharides (RFO) accumulation in developing chickpea (Cicer arietinum L.) seeds. Phytochemistry 125, 88–98 (2016).
Liu, J. J., Odegard, W. & De Lumen, B. Galactinol synthase from kidney bean cotyledon and zucchini leaf (purification and N-terminal sequences). Plant physiology 109, 505–511 (1995).
Saravitz, D. M., Pharr, D. M. & Carter, T. E. Galactinol synthase activity and soluble sugars in developing seeds of four soybean genotypes. Plant Physiology 83, 185–189 (1987).
Peterbauer, T. et al. Chain elongation of raffinose in pea seeds islation, charcterization, and molecular cloning of a multifunctional enzyme catalyzing the synthesis of stachyose and verbascose. Journal of Biological Chemistry 277, 194–200 (2002).
Smith, P. T., Kuo, T. M. & Crawford, C. G. Purification and characterization of galactinol synthase from mature zucchini squash leaves. Plant physiology 96, 693–698 (1991).
Peterbauer, T. & Richter, A. Biochemistry and physiology of raffinose family oligosaccharides and galactosyl cyclitols in seeds. Seed Science Research 11, 185–197 (2001).
dos Santos, T. B. et al. Expression of three galactinol synthase isoforms in Coffea arabica L. and accumulation of raffinose and stachyose in response to abiotic stresses. Plant Physiology and Biochemistry 49, 441–448 (2011).
Lahuta, L. B. et al. Dehydration induces expression of galactinol synthase and raffinose synthase in seedlings of pea (Pisum sativum L.). Journal of plant physiology 171, 1306–1314 (2014).
de Souza Vidigal, D. et al. Galactinol as marker for seed longevity. Plant Science 246, 112–118 (2016).
McCaskill, A. & Turgeon, R. Phloem loading in Verbascum phoeniceum L. depends on the synthesis of raffinose-family oligosaccharides. Proceedings of the National Academy of Sciences 104, 19619–19624 (2007).
Unda, F. et al. Isolation and characterization of galactinol synthases from hybrid poplar. Journal of experimental botany 63, 2059–2069 (2012).
Zhou, J. et al. Responses of Populus trichocarpa galactinol synthase genes to abiotic stresses. Journal of plant research 127, 347–358 (2014).
Downie, B. et al. Expression of a galactinol synthase gene in tomato seeds is up-regulated before maturation desiccation and again after imbibition whenever radicle protrusion is prevented. Plant Physiology 131, 1347–1359 (2003).
Li, X. et al. Expression of a galactinol synthase gene is positively associated with desiccation tolerance of Brassica napus seeds during development. Journal of plant physiology 168, 1761–1770 (2011).
Gojło, E. et al. The acquisition of desiccation tolerance in developing Vicia hirsuta seeds coincides with an increase in galactinol synthase expression and soluble α-d-galactosides accumulation. Journal of plant physiology 184, 37–48 (2015).
Carmi, N. et al. Cloning and functional expression of alkaline α-galactosidase from melon fruit: similarity to plant SIP proteins uncovers a novel family of plant glycosyl hydrolases. The Plant Journal 33, 97–106 (2003).
Dai, N. et al. Cloning and expression analysis of a UDP-galactose/glucose pyrophosphorylase from melon fruit provides evidence for the major metabolic pathway of galactose metabolism in raffinose oligosaccharide metabolizing plants. Plant physiology 142, 294–304 (2006).
Pillet, J. et al. VvGOLS1 and VvHsfA2 are involved in the heat stress responses in grapevine berries. Plant and Cell Physiology 53, 1776–1792 (2012).
Cheng, S. Ammopiptanthus Cheng f. A new genus of Leguminosae from central Asia. Journal of Botany USSR 44, 1381–1386 (1959).
Yan, S., Mu, G. & Xu, Y. Quaternary environmental evolution of the Lop Nur region, NW China. Acta Micropalaeontologica Sinica 17, 165–169 (1999).
Peters, S. et al. Protection mechanisms in the resurrection plant Xerophyta viscosa (Baker): both sucrose and raffinose family oligosaccharides (RFOs) accumulate in leaves in response to water deficit. Journal of Experimental Botany 58, 1947–1956 (2007).
Wang, L. et al. A role for a cell wall localized glycine-rich protein in dehydration and rehydration of the resurrection plant Boea hygrometrica. Plant Biology 11, 837–848 (2009).
Zhou, M.-L. et al. Genome-wide identification of genes involved in raffinose metabolism in Maize. Glycobiology 22, 1775–1785 (2012).
Panikulangara, T. J. et al. Galactinol synthase1. A novel heat shock factor target gene responsible for heat-induced synthesis of raffinose family oligosaccharides in Arabidopsis. Plant physiology 136, 3148–3158 (2004).
Peters, S. et al. Functional identification of Arabidopsis AtSIP2 (At3g57520) as an alkaline α-galactosidase with a substrate specificity for raffinose and an apparent sink-specific expression pattern. Plant and cell physiology 51, 1815–1819 (2010).
Knaupp, M. et al. Evidence for a role of raffinose in stabilizing photosystem II during freeze–thaw cycles. Planta 234, 477–486 (2011).
Gilmour, S. J. et al. Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant physiology 124, 1854–1865 (2000).
Jaglo-Ottosen, K. R. et al. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280, 104–106 (1998).
Kasuga, M. et al. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nature biotechnology 17, 287–291 (1999).
Liu, Q. et al. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought-and low-temperature-responsive gene expression, respectively, in Arabidopsis. The Plant Cell 10, 1391–1406 (1998).
Nishizawa, A., Yabuta, Y. & Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant physiology 147, 1251–1263 (2008).
Kim, M. S. et al. Galactinol is a signaling component of the induced systemic resistance caused by Pseudomonas chlororaphis O6 root colonization. Molecular Plant-microbe Interactions. 21, 1643–1653 (2008).
Zhou, T., Zhang, R. & Guo, S. Molecular cloning and characterization of GhGolS1, a novel gene encoding galactinol synthase from cotton (Gossypium hirsutum). Plant Molecular Biology Reporter 30, 699–709 (2012).
Zhao, T. Y. et al. Expression of the maize galactinol synthase gene family:(I) Expression of two different genes during seed development and germination. Physiologia Plantarum 121, 634–646 (2004).
Zhuo, C. et al. A cold responsive galactinol synthase gene from Medicago falcata (MfGolS1) is induced by myo-inositol and confers multiple tolerances to abiotic stresses. Physiologia plantarum 149, 67–78 (2013).
Song, J. et al. Cloning of galactinol synthase gene from Ammopiptanthus mongolicus and its expression in transgenic Photinia serrulata plants. Gene 513, 118–127 (2013).
Bachmann, M., Matile, P. & Keller, F. Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L.(cold acclimation, translocation, and sink to source transition: discovery of chain elongation enzyme). Plant physiology 105, 1335–1345 (1994).
Sprenger, N. & Keller, F. Allocation of raffinose family oligosaccharides to transport and storage pools in Ajuga reptans: the roles of two distinct galactinol synthases. The Plant Journal 21, 249–258 (2000).
Peterbauer, T., Puschenreiter, M. & Richter, A. Metabolism of galactosylononitol in seeds of Vigna umbellata. Plant and Cell Physiology 39, 334–341 (1998).
Peterbauer, T. et al. Stachyose synthesis in seeds of adzuki bean (Vigna angularis): molecular cloning and functional expression of stachyose synthase. The Plant Journal 20, 509–518 (1999).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic acids research 22, 4673–4680 (1994).
Nicholas, K. B., Nicholas, H. & Deerfield, D. GeneDoc: analysis and visualization of genetic variation. Embnew. news 4 (1997).
Tamura, K. et al. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular biology and evolution 24, 1596–1599 (2007).
Saitou, N. & Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular biology and evolution 4, 406–425 (1987).
Arnold, K. et al. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201 (2006).
Schwede, T. et al. SWISS-MODEL: an automated protein homology-modeling server. Nucleic acids research 31, 3381–3385 (2003).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic acids research 29, e45–e45 (2001).
Pirttilä, A. M. et al. Detection of intracellular bacteria in the buds of Scotch pine (Pinus sylvestris L.) by in situ hybridization. Applied and Environmental Microbiology 66, 3073–3077 (2000).
Acknowledgements
This work was supported by the China Agriculture Research System (Grant No.: CARS-25), the Major Scientific Research Projects of Liaoning Province (Grant No.: 2014215011) and the Fundamental Research Program of key laboratory of Education Bureau of Liaoning Province, China (Grant No.: LZ2015064)
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Y.L., L.Z. and M.Q. Performed the experiments: Y.L., L.Z., L.C. and H.M. Analyzed the data: Y.R., T.X., Y.H. and C.X. Wrote the paper: Y.L., L.Z. and M.Q. Revised and approved the final version of the paper: Y.L. and M.Q.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liu, Y., Zhang, L., Chen, L. et al. Molecular cloning and expression of an encoding galactinol synthase gene (AnGolS1) in seedling of Ammopiptanthus nanus. Sci Rep 6, 36113 (2016). https://doi.org/10.1038/srep36113
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36113
This article is cited by
-
Comparative efficacy of bio-selenium nanoparticles and sodium selenite on morpho-physiochemical attributes under normal and salt stress conditions, besides selenium detoxification pathways in Brassica napus L.
Journal of Nanobiotechnology (2022)
-
Magnaporthe oryzae systemic defense trigger 1 (MoSDT1)-mediated metabolites regulate defense response in Rice
BMC Plant Biology (2021)
-
Comparative transcriptomics provides novel insights into the mechanisms of selenium tolerance in the hyperaccumulator plant Cardamine hupingshanensis
Scientific Reports (2018)
-
Combined analysis of mRNA and miRNA identifies dehydration and salinity responsive key molecular players in citrus roots
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.