Abstract
Single-photon emission computed tomography combined with micro-CT (SPECT/μCT) imaging using Nanobodies against complement receptor of the Ig superfamily (CRIg), found on tissue macrophages such as synovial macrophages, has promising potential to visualize joint inflammation in experimental arthritis. Here, we further addressed the specificity and assessed the potential for arthritis monitoring. Signals obtained with 99mTc-labelled NbV4m119 Nanobody were compared in joints of wild type (WT) versus CRIg−/− mice with collagen-induced arthritis (CIA) or K/BxN serum transfer-induced arthritis (STIA). In addition, SPECT/μCT imaging was used to investigate arthritis development in STIA and in CIA under dexamethasone treatment. 99mTc-NbV4m119 accumulated in inflamed joints of WT, but not CRIg−/− mice with CIA and STIA. Development and spontaneous recovery of symptoms in STIA was reflected in initially increased and subsequently reduced joint accumulation of 99mTc-NbV4m119. Dexamethasone treatment of CIA mice reduced 99mTc-NbV4m119 accumulation as compared to saline control in most joints except knees. SPECT/μCT imaging with 99mTc-NbV4m119 allows specific assessment of inflammation in different arthritis models and provides complementary information to clinical scoring for quantitatively and non-invasively monitoring the pathological process and the efficacy of arthritis treatment.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic destructive inflammatory disease of the joints, featuring a mixed mononuclear and granulocytic cellular infiltration in the inflamed joints1. Over the past years, RA treatment has shifted from controlling symptoms with non-steroidal anti-inflammatory drugs and glucocorticoids such as dexamethasone, to controlling the disease process with anti-proliferative or cytotoxic drugs like methotrexate, alone or in combination with biologics2,3.
Rodent arthritis models have contributed greatly to the overall knowledge of RA and allow preclinical assessment of novel diagnostic and therapeutic interventions3. Type II collagen induced arthritis in DBA/1 mice (CIA) represents the current gold standard and has been used extensively for evaluation of novel therapies3. CIA requires specific disease-susceptible inbred mouse strains (DBA/1 and B10.RIII) and places a heavy emphasis on the early inductive phase of disease4. However, most transgenic and knockout strains of mice are in a C57BL/6J background (H-2b), which is regarded to be relatively resistant to arthritis induction5. In this context, it has been reported that C57BL/6J mice are indeed susceptible to arthritis induced by chicken type II collagen with a modified immunization protocol. The development of collagen induced arthritis is associated with a very strong T cell response that remains elevated for up to 3 months post-immunization6. KRN mice expressing the KRN transgenic T cell receptor and MHC class II molecule Ag7 develop severe inflammatory arthritis7. Transfer of arthritogenic serum of KRN and nonobese diabetic (K/BxN) mice leads to induction of robust and reproducible disease in several mouse strains (K/BxN serum-transfer-induced arthritis [STIA])8. Interestingly, the sterile inflammatory arthritis of STIA is transient, often spontaneously resolving after 15–30 days. Similar to human arthritis, STIA features high expression of pathogenic autoantibodies to glucose-6-phosphate isomerase (GPI), making this model ideal for the identification of the effector pathways involved in the arthritis process9.
Standard non-invasive readouts of disease severity such as paw thickness/volume or clinical scores mainly emphasize the oedema component of arthritic disease10. Other non-invasive readouts to monitor disease evolution or treatment efficacy, such as near-infrared, PET or SPECT imaging and scintigraphy, possibly combined with anatomical imaging techniques such as X-ray, ultrasonography and magnetic resonance imaging, can provide information on underlying molecular and cellular inflammatory processes as well as bone destruction. Tracers used for molecular arthritis imaging include 18F-fluoro-2-deoxy-D-glucose for visualizing glucose metabolism and 99mTc-methylenediphosphonate for monitoring bone turnover11. Various stroma-targeting tracers have been described for imaging arthritic synovitis, including 99mTc-anti-E-selectin-Fab for targeting activated endothelium12, radiolabelled antibodies targeting fibroblast activation protein for detection of fibrobolast-like synoviocytes13 or probes targeting activated macrophages such as 18F-polyethyleneglycol-folate14,15 or 111In-anti-F4/80-A3-116. In addition, radiolabeled biologicals such as anti-CD3, anti-CD4, anti-CD20 and anti-TNF have been evaluated for imaging RA13. As new tools being introduced in this field, Nanobodies are tracers combining the specificity of antibodies with the pharmacokinetics of small molecules, thus yielding high contract images within hours after inoculation17. In a preclinical setting, Nanobodies allow convenient SPECT imaging after standard tricarbonyl-chemistry-based labelling with 99mTc on their carboxy-terminal hexahistidine-tag18, distant from the antigen-binding site and thus not interfering with antigen recognition19.
In an approach of targeting markers expressed on macrophages for non-invasive inflammation tracking, we reported before that radiolabelled Nanobodies targeting the macrophage mannose receptor (NbMMR), initially described as effective probes for in vivo imaging of tumor-associated macrophages in hypoxic tumor areas20,21, also accumulate in joints of CIA mice22. Similarly, joint accumulation of the anti-CRIg Nanobody NbV4m119, as determined via SPECT/μCT imaging and quantification of radioactivity in dissected joints, correlates with arthritic scores and reflects disease severity over a range of scores in different joints23. Whereas osteoclasts and macrophages express similar levels of MMR22, CRIg is readily detected on macrophages but not osteoclasts (data not shown). Immunofluorescence microscopy has revealed that NbV4m119 specifically targets a subset of CD68-positive macrophages in synovium of DBA/1 CIA mice23. In line with a lower signal in non-arthritic joints, and thus a higher contrast for NbV4m119 as compared to NbMMR, accumulation of 99mTc-NbV4m119 in knees of mice developing CIA could even be detected before the onset of macroscopic clinical symptoms.
In concordance with CRIg expression on tissue macrophages such as Kupffer cells, 99mTc-NbV4m119 uptake in the liver was shown to be higher as compared to a non-targeting control Nanobody in WT but not CRIg−/− C57BL/6J mice23. In the current study, we further confirmed the specificity of CRIg targeting by NbV4m119 in arthritic joints of WT versus CRIg−/− C57BL/6J CIA mice. In addition, STIA was induced through transfer of arthritogenic serum of K/BxN mice, progeny of KRN mice, transgenic for a T cell receptor recognizing an epitope of bovine RNase, and nonobese diabetic mice7,8. Interestingly, the sterile inflammatory arthritis of STIA is transient, spontaneously resolving after 15–30 days9, thus allowing assessment of 99mTc-NbV4m119-based monitoring of the onset, development and recovery of arthritis symptoms of C57BL/6J STIA mice. Finally, we also used 99mTc-NbV4m119 to monitor dexamethasone treatment effects in DBA/1 CIA mice.
Results
Immunofluorescence microscopy to assess macrophage-specific NbV4m119 targeting in joints of collagen-induced arthritis mice
As a first step in addressing the target specificity at the level of the cell-type expressing CRIg that is being targeted by the NbV4m119, CIA was induced in DBA/1 mice and immunofluorescence microscopy was performed on tissue sections of decalcified ankles exhibiting different arthritic scores. Especially in sections of ankles exhibiting higher arthritic scores, staining by the NbV4m119 could be detected (see Supplementary Fig. S0). Moreover, the staining pattern of NbV4m119 overlapped with that one of an antibody targeting the macrophage marker F4/80, indicating that NbV4m119 is indeed binding to macrophages in the arthritic joints.
Specificity of NbV4m119 targeting CRIg in C57BL/6J mice with CIA
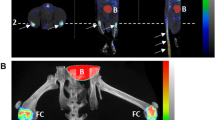
Another approach to assess specificity of targeting, is to compare targeting in WT versus CRIg−/− mice. Since CRIg−/− mice are not available in the DBA/1 background and C57BL/6J mice are relatively resistant to CIA5, we used a modified immunization protocol to induce CIA in WT and CRIg−/− C57BL/6J mice (Fig. 1a). Yet even when using this protocol, both WT and CRIg−/− C57BL/6J mice showed a low disease incidence (2 out of 7 mice developing symptoms in each group) as evaluated in two separate experiments and in fact, arthritic scores of individual paws tended to be even lower in CRIg−/− C57BL/6J as compared to WT C57BL/6J mice (see Supplementary Fig. S1a). As depicted in the experimental time lines in Fig. 1a, imaging using 99mTc-NbV4m119 was performed at day 34 in the four symptomatic mice and four asymptomatic mice (two WT and two CRIg−/− C57BL/6J mice). As had been documented and quantified before23, 99mTc-NbV4m119 targeted CRIg specifically in liver of naïve and arthritic WT mice, but not in CRIg−/− mice (see whole body images in Supplementary Fig. S1b). Signals that were detected for 99mTc-NbV4m119 in the kidneys and the bladder were also observed for a control, non-targeting 99mTc-NbBCII10 and are not attributable to specific targeting, but to the fast filtration of unbound Nanobody from the bloodstream through the renal route. As far as the uptake in joints is concerned, in WT mice 99mTc-NbV4m119 showed higher uptake in inflamed joints as compared to asymptomatic joints (Fig. 1b, left of the dotted line). In contrast, inflamed joints of CRIg−/− C57BL/6J mice (score 3 as compared to score 0) only showed a slightly higher uptake of 99mTc-NbV4m119 that may be due to joint oedema (Fig. 1b, left of the dotted line). Moreover, when comparing joints with the same arthritic score 3, 99mTc-NbV4m119 signals were much higher in WT versus CRIg−/− mice, indicating the antigen-specificity of these signals.
SPECT/CT quantification and imaging of 99mTc-NbV4m119, 99mTc-NbMMR or 99mTc-NbBCII10 accumulation in WT and CRIg−/− (KO) C57BL/6J mice with CIA after the onset of the arthritis symptoms.
(a) Experimental timelines for C57BL/6J mice with CIA; IFA = Incomplete Freund’s Adjuvant; M.t = Mycobacterium tuberculosis strain H37RA; Sac = mice were sacrificed. SPECT/micro-CT imaging was performed 3 h post intravenous injection of 99mTc-labeled NbV4m119, NbMMR or NbBCII10. (b) Signals obtained in the joints with score 3 and joints with score 0 were grouped in four symptomatic and four asymptomatic mice (two WT and two KO mice in each group). Data are expressed as mean values of % of injected activity (%IA) of 99mTc-Nanobodies obtained from the limbs (front paws, metatarsal joints and ankles). **p < 0.01 and ***p < 0.001. (c) Representative SPECT/μCT image of front paws in CIA mice, 3 h post injection with 99mTc-labeled NbV4m119, NbMMR or NbBCII10. Clinical scores are indicated next to each joint. Representative images of 1 mouse per group are shown using National Institutes of Health color scale and are scaled to maximum in the whole image. The data are representative of two independent experiments.
A reduced accumulation of NbV4m119 in CRIg−/− mice as compared to WT mice could not only be due to the absence of CRIg as a target in these mice, but could also be related to a functional effect of the absence of CRIg on the intensity of the arthritic inflammation or oedema that would affect the tissue penetration of Nanobodies in general, independent of the target specificity. To exclude this possibility we also included NbMMR in this study as a Nanobody of which the target is still expected to be present in CRIg−/− mice. Thereby, the uptake of 99mTc-NbMMR was quantified via SPECT/μCT imaging in the same mice at day 38 post immunization (see experimental time lines in Fig. 1a). As reported before22, NbMMR targets MMR in several organs and tissues, including myocardium, bone marrow, spleen and liver, but also accumulates in joints of CIA mice (see Supplementary Fig. S1b). Notably, and in contrast to the results obtained with 99mTc-NbV4m119, uptake of 99mTc-NbMMR was enhanced in inflamed versus asymptomatic joints in both WT mice and CRIg−/− C57BL/6J mice (Fig. 1b, right of the dotted line), arguing against a reduced joint inflammation in CRIg−/− CIA mice being the cause for the lower 99mTc-NbV4m119 uptake. Furthermore, reconstructed SPECT/μCT images revealed hot spots of radioactive signals only in symptomatic wrists in WT mice injected with 99mTc-NbV4m119 and WT or CRIg−/− mice injected with 99mTc-NbMMR, but not in 99mTc-NbV4m119 injected CRIg−/− mice or in mice injected with a control, non-targeting NbBCII10 (at day 42 post immunization), confirming specificity of the 99mTc-NbV4m119 signals (see cropped images focusing on front paws in Fig. 1c). Similar conclusions were obtained for signals in other joints such as ankles or knees (see whole body images in Supplementary Fig. S1c).
Specificity of NbV4m119 targeting in STIA C57BL/6J mice
Next, STIA was induced in C57BL/6J mice, whereby 8 days later one group of mice was used for imaging using 99mTc-NbV4m119 and another group of mice was used for imaging using 99mTc-NbMMR (see experimental time lines in Fig. 2a). Symptoms were mainly observed in the ankles, whereas some of the wrists seemed to remain asymptomatic at day 8 (see Supplementary Fig. S2a,S2b). For imaging with 99mTc-NbV4m119, 4 WT and 4 CRIg−/− serum injected mice at day 8 (all mice developing symptoms) were compared to 3 WT and 3 KO naive mice. The mean levels of 99mTc-NbV4m119 uptake in wrists and ankles of WT arthritic mice, which developed symptoms, were higher as compared to naive WT and CRIg−/− mice (Fig. 2a). The signal of 99mTc-NbV4m119 uptake in symptomatic wrists and ankles of CRIg−/− mice was not different as compared to non-symptomatic joints or naive joints in CRIg−/− mice. The same conclusions were obtained via measurement of radioactivity in dissected joints of euthanized mice as compared to quantification of the imaging data (see Supplementary Fig. S2c). Representative images cropped to focus on hind legs are shown in Fig. 2c, and whole body images including also front paws are shown in Supplementary Fig. S2e, highlighting focal signals of 99mTc-NbV4m119 uptake in ankles of arthritic WT mice, but not of naïve WT or arthritic or naïve CRIg−/− mice (Fig. 2c).
In vivo SPECT/CT imaging with an anti-CRIg tracer (NbV4m119) or an anti-MMR tracer in arthritic joints of C57BL/6J WT and CRIg−/− (KO) mice with STIA.
Experimental timelines and quantification of tracer accumulation in wrist and ankle joints of C57BL/6J mice with STIA which were injected with KBxN serum and monitored via SPECT/micro-CT imaging using 99mTc-NbV4m119 (a) or 99mTc-NbMMR (b) at day 8 post serum transfer. For each tracer, 4 KO and WT mice were injected with serum and 3 naïve KO and WT mice were used as control. Imaging & Sac = mice were imaged and sacrificed. The % of injected activity (%IA) was quantified for the wrist or ankle area in SPECT imaging (mean ± SEM, ***p < 0.001, ns: not significant). Naive: naive mice without serum injection; Score > 0: the ankles with arthritis score 1, 2 or 3. (c) Representative SPECT/μCT image of STIA C57BL/6J mice, 3 h post injection with 99mTc-labeled NbV4m119 or 99mTc-labeled NbMMR. Clinical scores are indicated next to each joint. Representative images of ankles in 1 out of 4 mice per group are shown using National Institutes of Health color scale and are scaled to maximum in the whole image.
A different set of mice was imaged with the 99mTc-NbMMR, whereby 4 WT and 4 CRIg−/− serum injected mice at day 8 were compared to 3 WT and 3 CRIg−/− naive mice. The wrists and ankles showed significantly higher signals in symptomatic WT mice (score > 0) as compared to naive WT mice (Fig. 2b,c and Supplementary Fig. S2d). Of note, in this experiment the symptomatic CRIg−/− mice had a lower score in ankles than the WT mice, suggesting CRIg deficiency may (variably) affect arthritis development in these mice (Supplementary Fig. S2b). Accordingly, 99mTc-NbMMR uptake was significantly higher in the ankle joints but not in wrists of symptomatic WT as compared to symptomatic CRIg−/− mice. Yet, in contrast to the results obtained with NbV4m119, NbMMR uptake in CRIg−/− mice was increased in ankles and wrists of symptomatic mice as compared to naive mice (Fig. 2b,c and Supplementary Fig. S2d,S2f), indicating that the lack of accumulation of 99mTc-NbV4m119 in ankles and wrists of symptomatic versus asymptomatic CRIg−/− mice truly reflects the absence of specific targeting to inflamed joints in the KO mice rather than merely reduced inflammation in joints of CRIg−/− mice.
Using 99mTc-NbV4m119 for monitoring arthritis progression in STIA
Next, we evaluated 99mTc-NbV4m119 for monitoring the initial induction and later recovery of RA symptoms in STIA. 12 C57BL/6J mice were injected with K/BxN serum on day 0. Mice were imaged with 99mTc-NbV4m119 at 2 days (onset of RA), 8 days (peak of RA) and 15 days (recovering phase of RA) after initial immunization (Fig. 3a). The scores of these mice increased from day 2 to day 8 and subsequently decreased after 15 days (Fig. 3b and Supplementary Fig. S3a). Significant 99mTc-NbV4m119 uptake was mainly observed in inflamed joints in hind legs (Fig. 3c) and in front paws (see Supplementary Fig. S3b) of arthritic mice. When assessing probe accumulation in individual wrists and ankles, 99mTc-NbV4m119 uptake at day 8 was significantly increased as compared to day 2 (Fig. 3d). In line with the observation that the symptoms were reduced in mice beyond day 8, the 99mTc-NbV4m119 accumulation in the wrists and ankles significantly decreased at day 15 as compared to day 8 (Fig. 3d). These results demonstrate that 99mTc-NbV4m119 can sense the CRIg level in vivo from the onset, peak and recovery of arthritis.
In vivo SPECT/CT imaging with an anti-CRIg tracer for monitoring arthritis recovery in STIA C57BL/6J mice.
(a) Experimental timelines for 12 WT mice injected with KBxN serum and imaged at day 2, day 8 and day 15 post serum transfer. Imaging = SPECT/μCT imaging. (b) The clinical arthritic scores (additive scores for all 4 paws of each mouse, expressed as group mean ± SEM) were measured at day 2, day 8 and day 15 after immunization. (c) Representative SPECT/μCT image of ankles in STIA serum transfer arthritis mice, 3 h post injection with 99mTc-labeled NbV4m119. The same arthritic mice displaying symptoms of arthritis showed specific uptake of 99mTc-labeled NbV4m119 in inflamed joints on day 2, day 8 and day 15 post serum injection. Clinical scores are indicated next to each joint. Representative images of 1 out of 12 mice per group are shown using National Institutes of Health color scale and are scaled to maximum in whole image. (d) Uptake of 99mTc-NbV4m119 in individual wrists and ankles of mice. The % of injected activity (%IA) was quantified for the wrist area. score 0: the wrists with score 0; S > 0: the wrists with arthritis score 1, 2 or 3. The % of injected activity (%IA) was quantified for the ankle area. (mean ± SEM, *P < 0.05, **P < 0.01, *** P< 0.001, ns: not significant).
Monitoring the effect of dexamethasone therapy for RA using 99mTc-NbV4m119 in CIA DBA/1 mice
Finally, we evaluated the use of 99mTc-NbV4m119 for monitoring the effects of therapeutic intervention. In order to obtain a maximum sensitivity for the effect of the therapeutic treatment, we used the gold-standard model of CIA in arthritis-sensitive DBA/1 mice for these experiments. Two groups of mice (n = 10) were compared to allow monitoring the effect of the dexamethasone treatment: one group received dexamethasone i.p. daily 28 days after arthritis-induction and the second group was injected with saline as control. Each group was imaged with 99mTc-NbV4m119 at day 28 (before treatment) and day 42 (after treatment) after initial immunization (Fig. 4a). Before the start of treatment at day 28, mice in both groups had an average cumulative arthritic score of 4. In contrast to the saline injected group, the scores of the mice in the dexamethasone treated group decreased to nearly baseline treatment till day 40. We also noticed that as soon as the dexamethasone treatment stopped on day 41, the arthritis scores of this group rebounded again on day 42 (Fig. 4b). The total 99mTc-NbV4m119 accumulation in four limbs of mice in the dexamethasone treated group (at 42 days post immunization) was significantly lower than in the saline treated group, which correlated with decreasing clinical scores (Fig. 4c,d). Similar conclusions were obtained in ankles, but no significant difference was detected among knees of dexamethasone treated and control mice. Hot spots of 99mTc-NbV4m119 accumulation were observed in knees of both groups of mice (see Supplementary Fig. S4). Notably, in all joints, the recorded imaging readout values in the dexamethasone injected group at day 42 remained at least as high as those recorded at day 28 (i.e. before the onset of therapy) (Fig. 4c).
Monitoring the clinical scores and uptake of 99mTc-NbV4m119 in the four limbs of DBA/1 mice with CIA following dexamethasone therapy.
(a) Experimental timelines for the CIA therapy monitoring experiment; CFA = Complete Freund’s Adjuvant; IFA = Incomplete Freund’s Adjuvant; Imaging = SPECT/μCT imaging; Sac = mice were sacrificed. Mice (n = 10) were immunized with type II collagen in complete Freund’s adjuvant. The treatments were started at 28 days and finished at 41 days after initial immunization. Treatments consisted of daily i.p. injection with 100 μl saline control or 0.25 mg/kg dexamethasone. (b) The clinical additive scores of the RA mice were measured until 42 days after immunization. *p < 0.05, **p < 0.01, and ***p < 0.001. (c) SPECT/CT image quantification of 99mTc-NbV4m119 in all 4 limbs of CIA DBA/1 mice (sum of ankles and front paws) at 28 days and 42 days after immunization were quantified as %IA. Data are expressed as mean ± SEM. ns: not significant, **p < 0.01. (d) Representative SPECT/μCT image of front paws in CIA DBA/1 mice, 3 h post injection with 99mTc-labeled NbV4m119. The same arthritic mice displaying symptoms of arthritis showed specific uptake of 99mTc-labeled NbV4m119 in front paws on 28 days and 42 days post serum injection. Clinical scores are indicated next to each joint. Representative images of front paws in 1 out of 10 per group CIA DBA/1 mice are shown using National Institutes of Health color scale and are scaled to maximum in whole image.
Discussion
Molecular imaging of arthritis, which includes anatomical imaging techniques such as ultrasonography and MRI or molecular imaging such as optical imaging or nuclear imaging, will improve the understanding of the pathogenesis and development of the disease. MRI imaging produces anatomically detailed images of cartilage, tendons, synovial membrane and bone which ultrasonography could not penetrate11. It provides a higher contrast resolution, allowing detection of synovial hyperplasia, cartilage and bone degradation, inflammation and osteitis and is able to show signs of early arthritis in patients24. Molecular imaging can provide molecular information on the underlying biochemical and inflammatory processes, that is complementary to anatomical imaging. Development of metal plasmonic nanoparticles for MRI25 allows using MRI also for molecular imaging in addition to its use for anatomical imaging. Other molecular probes being developed for molecular arthritis imaging include fluorescence and near-infrared labelled probes26 for optical imaging or radioisotopes labelled probes for scintigraphy, PET or SPECT14. Nuclear imaging techniques generate deeper tissue penetration and lower background than optical imaging techniques and are more sensitive than structural imaging11. In the current study, we built on previous results obtained with NbV4m119 as a probe for molecular imaging of arthritic inflammation23. Despite the low incidence of arthritis in the CIA model in C57BL/6J mice, we obtained antigen-specific 99mTc-NbV4m119 signals in arthritic WT mice but not in arthritic CRIg−/− mice. Also in the STIA model, which is readily inducible in C57BL/6J mice, 99mTc-NbV4m119 specifically detected the inflamed lesions in the arthritic WT mice but not in the CRIg−/− mice. Throughout these different experiments in the CIA and STIA models, hot spots of 99mTc-NbV4m119 accumulation in arthritic joints of CRIg−/− mice were never detected. Moreover, sustained joint accumulation of 99mTc-NbMMR in CRIg−/− mice indicates that the impaired accumulation of 99mTc-NbV4m119 in symptomatic CRIg−/− mice truly reflects the absence of specific targeting in CRIg−/− mice, rather than reduced joint inflammation.
99mTc-NbV4m119 signals provide a quantitative read-out for disease development and resolution in the STIA model. The detection of hot spots of NbV4m119 signal in some of the score 0 joints at day 2, suggests that the in vivo imaging is more sensitive than the classic scoring system. These findings offer perspectives for monitoring disease evolution, either spontaneous or under therapy, provided that baselines can be established allowing comparison of the obtained signals. Increased resolution when switching from SPECT to PET imaging and increased joint size in humans as compared to rodents, thereby hold potential for increased contrast upon clinical translation of such inflammation imaging.
Non-invasive imaging can monitor the same animal at different time points before or after the onset of arthritis, allowing to follow up a therapy or treatment. MRI-based outcome measures have recently been adopted by pharma companies for clinical trial research to measure clinical trial outcome of therapy such as anti-TNF agents, including adalimumab, infliximab, etanercept and golimumab. These enable earlier assessment of drug efficacy than radiographic outcomes27. In a recent study using 111In-labelled antibodies targeting fibroblast activation protein, macrophages and integrin αvβ3, decreased joint uptake of tracers was observed in arthritic mice undergoing etanercept treatment28. In the current study using 99mTc-NbV4m119 in CIA DBA/1 mice receiving dexamethasone as therapy, the recorded signals at day 42 were lower in the dexamethasone injected group as compared to the saline control group, but remained at least as high as those recorded at day 28 (i.e. before the onset of therapy). At the level of the knees, wherein 99mTc-NbV4m119-based imaging is a sensitive indicator of inflammation29, similar signals were even detected in the dexamethasone treated group as in the control group. These data are in line with the observation that the clinical scores rose immediately as soon as dexamethasone treatment stopped, confirming that dexamethasone is better at providing symptom relief than at altering disease progression30 and indicating that SPECT/μCT imaging can indeed provide complementary molecular information on the effect of the therapy on the underlying disease pathology in the various joints as compared to the clinical score.
Our studies have confirmed that Nanobodies targeting CRIg constitute a specific tool for non-invasive SPECT/CT imaging as a way of assessing inflammation in arthritis models in vivo. Moreover, the monitoring studies indicate that imaging arthritic mice using 99mTc-NbV4m119 appears to serve as a non-invasive means for tracing disease evolution and response to therapy, providing complementary and more sensitive information as compared to paw swelling and clinical scoring.
Materials and Methods
Ethical approval
Animal studies were performed according to the European Community Council Directive 2010/63/EU and were approved by our university’s Ethical Committee for Animal Experiments (Approval N°12-220-6; 24/09/2012).
Mice
Male C57BL/6J (8–12 week-old) and DBA/1 mice (8 week-old) were purchased from Janvier Sas. CRIg−/− mice (C57BL/6J background) were generously provided by Genentech (San Francisco, CA).
Arthritis models
CIA was induced in WT and CRIg−/− C57BL/6J male mice using a modified immunization involving two 21-day-separated subcutaneous injections of 100 μL of 4 mg/mL chicken collagen type II (Sigma-Aldrich) in acetic acid, emulsified in incomplete Freund’s adjuvant with added 3.3 mg/mL heat-killed Mycobacterium tuberculosis strain H37RA (Difco Laboratories), at two sites (the base of the tail and a slightly more anterior location) after anesthesia. Each limb was scored for severity of arthritis for CIA as follows: 0 = normal; 1 = redness/swelling of one joint; 2 = redness/swelling of more than one joint; 3 = swelling of entire paw; 4 = ankylosis and/or deformity. For dexamethasone treatment efficacy monitoring and immunofluorescence microscopy, cohorts of DBA/1 mice were used for CIA induction as described23 and were treated daily with i.p. 0.25 mg/kg dexamethasone in 100 μL saline or saline control from day 28 to day 41.
STIA mice were sensitized via an i.v. injection in the tail of C57BL/6J mice with 60 μL of arthritogenic serum from K/BxN mice as described8. All paws were assigned a score for inflammation in STIA on a scale of 0 to 3: 1 = mild swelling of the ankle insufficient to reverse the normal V shape of the foot; 2 = swelling sufficient to make the ankle and midfoot approximately equal in thickness to the forefoot; 3 = reversal of the normal V shape of the foot. The thickness of the ankles and the wrists were monitored using a calliper across the ankle or wrist joint at the widest point.
Nanobodies
Nanobodies against CRIg (NbV4m119), the β-lactamase BCII enzyme of Bacillus cereus (NbBCII10) and MMR (NbMMR) were generated as described19,22,23. For 99mTc labelling, the Nanobodies were subcloned into the pHEN6c vector introducing a hexahistidine-tag. All Nanobodies were produced in, and purified from, the periplasm of Escherichia coli WK6 cells.
99mTc-Nanobody labelling and pinhole SPECT/μCT analysis
Nanobodies were labelled with 99mTc via the hexahistidine tag, purified via NAP-5 column (Sephadex G-25, GE Healthcare) and 0,22 μm membrane filtered as described18. Radiochemical purity was determined using instant thin-layer chromatography (Pall Corp., Life Sciences) in acetone solvent, revealing the amount of Nb-complexed and unincorporated 99mTc at the application and the solvent front, respectively18. 52.8 ± 0.01 MBq of 99mTc-Nbs activity in 80–100 μL (at 192 MBq/nmol) was injected. 3 h post injection, anesthetized mice were imaged using μCT (Skyscan 1178; Skyscan) followed by pinhole SPECT (e.cam180; Siemens Medical Solutions) as described23. Images were reconstructed as described31, using an iterative reconstruction algorithm and corrected for attenuation and scatter. Micro-CT images were reconstructed using filtered backprojection (NRecon; SkyScan). Images were viewed and analyzed using AMIDE: A Medical Image Data Examiner software32. A calibration factor, obtained by SPECT image analyses of syringes with known activities, is used to convert SPECT image pixels to radioactivity levels. Regions of interest were drawn, recorded amounts of radioactivity in these regions were divided by the amount of injected activity (corrected for decay) and expressed as percentage of injected activity (% IA).
To determine biodistribution ex vivo, mice were sacrificed and tissues were dissected and weighed, and their radioactivity content was measured using an automated γ-counter (Cobra II Inspector 5003; Canberra-Packard, Schwadorf, Austria). Organ uptake was calculated as the percentage of injected activity per gram (% IA/g) and corrected for decay.
SPECT/μCT imaging time lines for the different arthritis models
(1) In vivo imaging monitoring the modified CIA model in WT (n = 2) and CRIg−/− (n = 2) C57BL/6J mice. The imaging was performed at day 34, day 38 and day 42, using 99mTc-NbV4m119, 99mTc-NbMMR, or 99mTc-NbBCII10 respectively. The clinical scores of the wrists and ankles were monitored. (2) In vivo imaging monitoring STIA at day 8. WT and CRIg−/− C57BL/6J mice (n = 4, 14 weeks old) received the KBxN mouse serum administration. The clinical scores and the thickness of the wrists and ankles were monitored at day 8. The arthritic WT and CRIg−/− mice were injected with 99mTc-NbV4m119 or 99mTc-NbMMR and imaged by SPECT/CT on day 8 post serum injection. After the anesthetized mice were imaged using μCT followed by pinhole SPECT, mice were sacrificed, wrists and ankles were dissected and weighed, and their radioactivity content was measured using an automated γ-counter. (3) In vivo imaging monitoring the STIA pathological process. WT C57BL/6J mice (n = 12, 10 weeks old) received the KBxN mice serum administration. The clinical scores and the thickness of the wrists and ankles were monitored till day 15. The arthritic WT C57BL/6J mice were injected with 99mTc-NbV4m119 each time and imaged by SPECT/CT on day 2 (onset of RA), day 8 (peak of RA) and day 15 (RA partly resolved) post serum injection. Anesthetized mice were imaged using μCT followed by pinhole SPECT. (4) Treatment efficacy monitoring by 99mTc-NbV4m119. For evaluation of the dexamethasone therapy by imaging, beginning on 28 days after the initial immunization, cohorts of CIA DBA-1 mice (n = 10) were treated i.p. with dexamethasone (daily) or received saline (daily) and served as control. The treatments were finished at day 41. Till day 42, the scores were monitored for each mouse every two days. The arthritic mice were injected with 99mTc-NbV4m119 and imaged by SPECT/CT before (day 28) and after (day 42) the dexamethasone or saline treatments.
Immunofluorescence microscopy
CIA was induced in DBA/1 mice. Mice were sacrificed under anesthesia and ankles exhibiting different arthritic scores were removed and fixed in 4% paraformaldehyde solution for 24 hours. Ankles were decalcified in 10% EDTA pH 7.4 (Sigma, USA) for 4 weeks (EDTA solution refreshed once a week) then embedded in paraffin. 8 μm sections were prepared from paraffin-embedded tissues. The sections were incubated in 1% blocking reagent (Roche) in PBS (1% PBS/BR) containing detection antibodies. Anti-F4/80(Cl:A3-1)/Alexa fluor 488 was purchased from AbD Serotec and anti-HA-tag (16B12)/Alexa Fluor 594 was purchased from Invitrogen. Each section was incubated overnight with 2 μg of antibody and Nb. Specific labelling was detected with anti-HA/Alexa fluor 549 in 1% PBS-BR. Fluoro-Gel II/DAPI (Electron Microscopy Sciences) mounted slides were used for fluorescence microscopy with a UPlanFI 10x, 20x or 30x objective on a OLYMPUS BX51 microscope with OLYMPUS DP71 CCD and OLYMPUS DP Controller software.
Statistical analysis
Significance analysis of data was conducted using a two-tailed unpaired Student’s T test when two groups were analyzed or one-way ANOVA analysis of variance multiple–comparison post-test. Prism 6.0 (Graphpad software) was used for statistical analyses and graph creation. P-values ≤ 0.05 were considered significant.
Additional Information
How to cite this article: Zheng, F. et al. Specificity Evaluation and Disease Monitoring in Arthritis Imaging with Complement Receptor of the Ig superfamily targeting Nanobodies. Sci. Rep. 6, 35966; doi: 10.1038/srep35966 (2016).
References
Klareskog, L., Malmstrom, V., Lundberg, K., Padyukov, L. & Alfredsson, L. Smoking, citrullination and genetic variability in the immunopathogenesis of rheumatoid arthritis. Seminars in immunology 23, 92–98, doi: 10.1016/j.smim.2011.01.014 (2011).
Colmegna, I., Ohata, B. R. & Menard, H. A. Current understanding of rheumatoid arthritis therapy. Clinical pharmacology and therapeutics 91, 607–620, doi: 10.1038/clpt.2011.325 (2012).
Upchurch, K. S. & Kay, J. Evolution of treatment for rheumatoid arthritis. Rheumatology 51 Suppl 6, vi28–vi36, doi: 10.1093/rheumatology/kes278 (2012).
Jirholt, J. et al. Genetic linkage analysis of collagen-induced arthritis in the mouse. Eur J Immunol 28, 3321–3328, doi: 10.1002/(SICI)1521-4141(199810)28:10<3321::AID-IMMU3321>3.0.CO;2-M (1998).
Inglis, J. J., Simelyte, E., McCann, F. E., Criado, G. & Williams, R. O. Protocol for the induction of arthritis in C57BL/6 mice. Nat Protoc 3, 612–618, doi: 10.1038/nprot.2008.19 (2008).
Geboes, L. et al. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum 60, 390–395, doi: 10.1002/art.24220 (2009).
Ditzel, H. J. The K/BxN mouse: a model of human inflammatory arthritis. Trends Mol Med 10, 40–45 (2004).
Misharin, A. V. et al. Nonclassical Ly6C(−) monocytes drive the development of inflammatory arthritis in mice. Cell Rep 9, 591–604, doi: 10.1016/j.celrep.2014.09.032 (2014).
Monach, P. A., Mathis, D. & Benoist, C. The K/BxN arthritis model. Curr Protoc Immunol Chapter 15, Unit 15 22, doi: 10.1002/0471142735.im1522s81 (2008).
Arnett, H. A. & Viney, J. L. Considerations for the sensible use of rodent models of inflammatory disease in predicting efficacy of new biological therapeutics in the clinic. Advanced drug delivery reviews 59, 1084–1092, doi: 10.1016/j.addr.2007.06.013 (2007).
McQueen, F. M. MRI in rheumatoid arthritis: a useful tool for the clinician? Postgraduate medical journal 90, 332–339, doi: 10.1136/postgradmedj-2013-132121 (2014).
Jamar, F. et al. Scintigraphy using a technetium 99m-labelled anti-E-selectin Fab fragment in rheumatoid arthritis. Rheumatology (Oxford) 41, 53–61 (2002).
Laverman, P. et al. Immuno-PET and Immuno-SPECT of Rheumatoid Arthritis with Radiolabeled Anti-Fibroblast Activation Protein Antibody Correlates with Severity of Arthritis. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 56, 778–783, doi: 10.2967/jnumed.114.152959 (2015).
Gandjbakhch, F. et al. Determining a magnetic resonance imaging inflammatory activity acceptable state without subsequent radiographic progression in rheumatoid arthritis: results from a followup MRI study of 254 patients in clinical remission or low disease activity. The Journal of rheumatology 41, 398–406, doi: 10.3899/jrheum.131088 (2014).
Matteson, E. L. et al. Assessment of disease activity in rheumatoid arthritis using a novel folate targeted radiopharmaceutical Folatescan. Clinical and experimental rheumatology 27, 253–259 (2009).
Terry, S. Y. et al. (1)(1)(1)In-anti-F4/80-A3-1 antibody: a novel tracer to image macrophages. European journal of nuclear medicine and molecular imaging 42, 1430–1438, doi: 10.1007/s00259-015-3084-8 (2015).
Schoonooghe, S. et al. Novel applications of nanobodies for in vivo bio-imaging of inflamed tissues in inflammatory diseases and cancer. Immunobiology 217, 1266–1272, doi: S0171-2985(12)00173-8 [pii] 10.1016/j.imbio.2012.07.009 (2012).
Xavier, C. et al. Site-specific labeling of his-tagged Nanobodies with (9)(9)mTc: a practical guide. Methods Mol Biol 911, 485–490, doi: 10.1007/978-1-61779-968-6_30 (2012).
Vincke, C. et al. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J Biol Chem 284, 3273–3284 (2009).
Movahedi, K. et al. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res 72, 4165–4177, doi: 10.1158/0008-5472.CAN-11-2994 (2012).
Blykers, A. et al. PET Imaging of Macrophage Mannose Receptor-Expressing Macrophages in Tumor Stroma Using 18F-Radiolabeled Camelid Single-Domain Antibody Fragments. J Nucl Med 56, 1265–1271, doi: 10.2967/jnumed.115.156828 (2015).
Put, S. et al. SPECT imaging of joint inflammation with Nanobodies targeting the macrophage mannose receptor in a mouse model for rheumatoid arthritis. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 54, 807–814, doi: 10.2967/jnumed.112.111781 (2013).
McQueen, F. M. et al. MRI osteitis predicts cartilage damage at the wrist in RA: a three-year prospective 3T MRI study examining cartilage damage. Arthritis research & therapy 16, R33, doi: 10.1186/ar4462 (2014).
McQueen, F. M. Imaging in early rheumatoid arthritis. Best practice & research. Clinical rheumatology 27, 499–522, doi: 10.1016/j.berh.2013.09.005 (2013).
Popovich, I., Dalbeth, N., Doyle, A., Reeves, Q. & McQueen, F. M. Exploring cartilage damage in gout using 3-T MRI: distribution and associations with joint inflammation and tophus deposition. Skeletal radiology 43, 917–924, doi: 10.1007/s00256-014-1869-7 (2014).
Gompels, L. L. et al. In vivo fluorescence imaging of E-selectin: quantitative detection of endothelial activation in a mouse model of arthritis. Arthritis Rheum 63, 107–117, doi: 10.1002/art.30082 (2011).
McQueen, F. M. & Chan, E. Insights into rheumatoid arthritis from use of MRI. Current rheumatology reports 16, 388, doi: 10.1007/s11926-013-0388-1 (2014).
Terry, S. Y. et al. Monitoring Therapy Response of Experimental Arthritis with Radiolabeled Tracers Targeting Fibroblasts, Macrophages, or Integrin alphavbeta3. J Nucl Med 57, 467–472, doi: 10.2967/jnumed.115.162628 (2016).
Zheng, F. et al. Monitoring liver macrophages using nanobodies targeting Vsig4: concanavalin A induced acute hepatitis as paradigm. Immunobiology 220, 200–209, doi: 10.1016/j.imbio.2014.09.018 (2015).
Kang, I., Lee, W. W. & Lee, Y. Modulation of collagen-induced arthritis by IL-4 and dexamethasone: the synergistic effect of IL-4 and dexamethasone on the resolution of CIA. Immunopharmacology 49, 317–324 (2000).
Vanhove, C., Defrise, M., Bossuyt, A. & Lahoutte, T. Improved quantification in single-pinhole and multiple-pinhole SPECT using micro-CT information. European journal of nuclear medicine and molecular imaging 36, 1049–1063 (2009).
Loening, A. M. & Gambhir, S. S. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2, 131–137 (2003).
Acknowledgements
We thank Cindy Peleman for technical assistance. This project was financially supported by the Research Foundation Flanders (FWO-Vlaanderen), the Flanders Government Agency for Innovation by Science and Technology (IWT), the Belgian Interuniversity Attraction Poles, the Concerted Research Actions of the Regional Government of Flanders and by the National Natural Science Foundation of China (81501527). TL is senior clinical investigator for the Research Foundation Flanders and the National Fund for Scientific Research-Flanders. The work has been supported by a scholarship from the Top-Notch Student Scholarship Fund of the China Scholarship Council to FZ.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Z.F. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: F.Z., H.P., P.M., T.L., S.M., P.D.B., N.D., S.S. and G.R. Acquisition of data: F.Z., N.D. and S.S. Analysis and interpretation of data: F.Z., Y.W., S.L., P.D.B., N.D., S.S. and G.R.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zheng, F., Perlman, H., Matthys, P. et al. Specificity Evaluation and Disease Monitoring in Arthritis Imaging with Complement Receptor of the Ig superfamily targeting Nanobodies. Sci Rep 6, 35966 (2016). https://doi.org/10.1038/srep35966
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35966
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.