Abstract
Brucella abortus is an intracellular bacterial pathogen and an etiological agent of the zoonotic disease known as brucellosis. Brucellosis can be challenging to treat with conventional antibiotic therapies and, in some cases, may develop into a debilitating and life-threatening chronic illness. We used multiple independent assays of in vitro metabolism and intracellular replication to screen a library of 480 known bioactive compounds for novel B. abortus anti-infectives. Eighteen non-cytotoxic compounds specifically inhibited B. abortus replication in the intracellular niche, which suggests these molecules function by targeting host cell processes. Twenty-six compounds inhibited B. abortus metabolism in axenic culture, thirteen of which are non-cytotoxic to human host cells and attenuate B. abortus replication in the intracellular niche. The most potent non-cytotoxic inhibitors of intracellular replication reduce B. abortus metabolism in axenic culture and perturb features of mammalian cellular biology including mitochondrial function and receptor tyrosine kinase signaling. The efficacy of these molecules as inhibitors of B. abortus replication in the intracellular niche suggests “dual-target” compounds that coordinately perturb host and pathogen are promising candidates for development of improved therapeutics for intracellular infections.
Similar content being viewed by others
Introduction
Approximately 500,000 new cases of human brucellosis are reported annually1. However, this number does not fully reflect the total number of cases globally, as the disease remains undiagnosed or misdiagnosed in many areas of Asia, Africa, and South America where it inflicts a significant health, economic, and social burden2,3. As intracellular pathogens, Brucella spp. stably inhabit phagocytes and other host cells, which facilitates successful evasion of the host immune response and has the additional consequence of buffering the cells against antimicrobial compounds. Patients infected with Brucella require a one- to three-month course of treatment involving multiple antimicrobial agents4,5. Even after this extended treatment, the reported incidence of relapse ranges from 3–40%, depending on the course of therapy6. The prolonged treatment regimens required to clear Brucella infection often have adverse side effects in the patient including hepatotoxicity and gastric damage5.
Given that there is no approved human vaccine for brucellosis and that current antimicrobial treatments are long and often harmful to patients, the development of improved treatment strategies for this disease is a high priority. The initial goal of this study was to identify sets of small molecules that either a) directly or indirectly inhibit entry and/or replication of B. abortus in human macrophages by targeting host factors or b) directly inhibit metabolic activity of Brucella abortus in defined culture medium. Our host-targeted screen of 480 bioactive molecules from the ICCB chemical library identified 18 molecules that specifically inhibited B. abortus replication in the intracellular niche of a human cell line. We further identified 26 pathogen-targeting compounds that inhibited B. abortus metabolic activity in axenic culture. The most potent inhibitors of B. abortus in the intracellular niche inhibit B. abortus in axenic culture and have documented activities against host cells. We conclude that coordinate targeting of host and pathogen pathways may improve the efficacy of treatment of brucellosis and other intracellular bacterial infections.
Methods
Bacterial strains
All studies on live B. abortus strain 2308 were conducted at Biosafety Level 3 (BSL3) at the University of Chicago, Howard Taylor Ricketts Regional Biocontainment Laboratory according to US Federal Select Agent Program guidelines. mCherry B. abortus was previously generated from the wild-type B. abortus 2308 parent strain by integration of miniTn7 expressing a single copy mCherry at the glmS locus7.
Identification of ICCB compounds that inhibit B. abortus metabolism in axenic culture
Prior to compound screening, B. abortus 2308 was streaked out and cultivated at 37 °C and 5% CO2 for 48 hours on Schaedler blood agar (SBA) plates, re-streaked, and grown for another 48 hours. Cells were scraped off plates and suspended in 1X IF10b medium (Biolog). Cell concentration was adjusted to 5% transmittance at OD600 in 1X IF10b medium and diluted by a factor of 1:6.8 in PM9 inoculating solution: 2 mM MgCl2•6H2O, 1 mM CaCl2•2H2O, 0.005% yeast extract, 0.005% Tween 40, 2.5 mM D-glucose, 5 mM sodium pyruvate, 1X Dye Mix G (Biolog). Bacterial cell suspension was distributed to each well across six half-area 96-well plates at 50 μL per well. Drugs from the ICCB BioActives library (Enzo Life Sciences) were diluted 1:150 in PM9 inoculating solution and were mixed with cells at a final volume of 100 μL per well resulting in a final drug dilution of 1:300 and bacterial density equivalent to 65% transmittance. Plates were incubated at 37 °C and 5% CO2 for two days prior to measuring cell respiration (by assessing reduction of tetrazolium dye mix G at 630 nm) using a Tecan Infinite 200 PRO microplate reader. Compounds that led to a decrease in cell respiration one standard deviation below the mean were deemed hits.
Identification of compounds structure similarity
The Tanimoto similarity score of assayed drug compounds was obtained using a Chemical Structure Clustering Tool8. A Tanimoto similarity score of more than 0.68 was considered statistically significant (i.e. more than two standard deviations away from average similarity score calculated from 50 million compound pairs9).
Mammalian tissue culture and screen for compounds that inhibit B. abortus intracellular growth
THP-1 macrophage-like cells were grown to a maximum density of 1 × 106/mL in complete RPMI-1640 medium, 2 mM glutamine (Gibco), and 10% heat-inactivated fetal bovine serum (HyClone) at 37 °C in a humidified environment with 5% CO2. Three days prior to infection, THP-1 cells (100 μL aliquots at 1 × 106 cells/mL) were transferred to 96-well plates (Costar) and differentiated with 40 ng/mL phorbol myristate acetate (PMA). After 3 days of differentiation, the medium bathing the confluent cell monolayers was removed by pipetting and replaced with fresh medium. One day prior to infection, mCherry B. abortus 2308 was grown from frozen stocks in Brucella broth in a shaking incubator at 37 °C, washed, density quantified, and added at multiplicity of infection (MOI) 100 to wells of PMA-differentiated THP-1 cells containing either ICCB drugs (pre-infection treatment) or no drugs (post-infection treatment). In the case of pre-infection treatment, THP-1 cells were incubated with ICCB compounds for 2 hours prior to infection. Tissue culture plates were centrifuged at 2170 rpm for 10 minutes to promote and synchronize infection and then incubated for 1 hour at 37 °C. After this period, 100 μg/mL gentamicin was added to the wells for 1 hour to kill extracellular but not intracellular B. abortus. For pre-infection treatment experiments, culture medium was again removed and replaced with fresh medium containing ICCB compounds and 25 μg/mL gentamicin. For post-infection treatment experiments, culture medium was removed and replaced with medium containing 25 μg/mL gentamicin; ICCB compounds were added 4 hours later. Cells were incubated in the presence of ICCB compounds for 3 days before measuring mCherry B. abortus fluorescence using a Tecan Infinite 200 PRO microplate reader. Compounds that decreased intracellular fluorescence by ≥20% as normalized to dimethyl sulfoxide (DMSO)-treated control were considered hits.
Cytotoxicity assays of test compounds against mammalian cells
Two assays were used to assess cytotoxicity: MTT assay and enumeration of host cell nuclei. Reduction of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was used to measure mammalian (THP-1) cell viability in the presence of all assayed compounds at the end of a three-day incubation period with each of the compounds. MTT was added to cells at a final concentration of 0.5 mg/ml and the cells incubated for 4 hours at 37 °C in a humidified incubator at 5% CO2. The medium was replaced with 100 μL isopropyl alcohol containing 40 mM HCl and 20 μL of 10% sodium dodecyl sulfate to solubilize precipitated serum protein. Absorbance of the reaction product was measured at 570 nm in a Tecan Infinite 200 PRO microplate reader. Compounds that decreased reduction of tetrazolium dye by ≥20% of control in an average of two independent experiments were deemed cytotoxic. As a second cytotoxicity assay, we used the established method of nuclei counts per field of view. Briefly, we measured the number of Hoechst-stained nuclei per image in Cell Profiler (v2.1.1) and expressed the nuclei number as an average count from ≈7 images per treatment ± standard deviation from a single experiment.
Evaluation of morphological changes of THP-1 cells
To determine the effect of compounds on cell morphology, THP-1 cells were treated with compounds as described above and morphological changes were assessed by phase contrast imaging. Compounds deemed toxic induced morphological changes to THP-1 cells identified as cell rounding and shrinkage (Supplementary Fig. S1).
Quantification of B. abortus colony forming units
The effect of drug treatment on B. abortus intracellular replication, as assessed by enumeration of colony forming units (CFU), was assayed as follows. 3 days prior to experiment, THP-1 monocytic cells were seeded at 5 × 104 cells per well in a 96-well plate in the presence of 40 ng/mL PMA. One day prior to experiment, B. abortus 2308 was grown from frozen stocks in Brucella broth in a shaking incubator at 37 °C, washed, density quantified, and added at MOI 100 to wells of differentiated THP-1 cells that were treated with compounds 2 hours prior to, but not during infection. The plates were centrifuged at 2170 rpm for 10 minutes to promote and synchronize infection and then incubated for 1 hour at 37 °C. At that time, 100 μg/ml gentamicin was added for additional hour to kill extracellular, but not intracellular bacteria. After the incubation, medium was removed and replaced with fresh ICCB compounds and 25 μg/mL gentamicin. Cells were incubated in the presence of test compounds for 3 days. After incubation medium was removed and replaced with 200 μL of 0.1% Triton X-100/PBS. The cells were incubated for 10 minutes, mixed by pipetting, and serially diluted in PBS. 10 μL of each serial dilution was spotted on tryptic soy agar plates and incubated for 4 days prior to CFU counting. The results are expressed as an average of 3 to 7 biological replicates.
Imaging Brucella-infected THP-1 cells
THP-1 cells were seeded in a 96-well plate (Greiner), prepared, treated with compounds, and infected with B. abortus as described above. After incubation with final candidate compounds, cells were washed three times with 1X PBS and fixed by two 10-minute incubations with 4% paraformaldehyde followed by three washes with 1X PBS. Cell nuclei were labeled with 1 μg/mL Hoechst stain. Cells were imaged using a Leica-DMI6000B fluorescence microscope equipped with a 20X/0.4 NA objective. 6–24 images were captured per each condition using Leica Application Suite X and Hamamatsu Orca-R2 camera. THP-1 nuclei were enumerated to assess compound cytotoxicity in the context of B. abortus infection. As described above, Cell Profiler (v2.1.1) was used to measure the number of Hoechst-stained nuclei per image; the number of nuclei was expressed as an average count from ≈7 images per treatment ± standard deviation. The number of intracellular fluorescent brucellae was enumerated using the same approach. The effect of the compounds on B. abortus intracellular growth was assessed by calculating the number of bacteria per cell represented as a ratio of the total number of bacteria to the total number of nuclei (brucellae per nucleus) per image calculated as an average from ≈7 images per treatment ± standard deviation.
Determination of minimum inhibitory concentration (MIC)
All compounds used to determine MICs were obtained from Santa Cruz Biotechnology except 1400W, which was obtained from Cayman Chemicals. Stock solutions for compounds were prepared by reconstitution in DMSO except for 1400W, which was reconstituted in water. All compound solutions were prepared fresh prior to experiments. MICs of selected compounds were determined by pre-treating cells 2 hours prior to infection. After a 2-hour pre-treatment, cells were infected with B. abortus at 100 MOI, cultured for 3 days in the presence of compounds, fixed, stained, and imaged as described above. The number of intracellular bacteria were quantified relative to the number of nuclei using Cell Profiler. MICs represent concentrations of compounds that significantly inhibited number of intracellular B. abortus (p < 0.05) at that specific concentration in at least two of four independent experiments. Statistical significance of MICs was determined from an average of ten images from each of the four biological replicates using one-way ANOVA followed by Dunnett’s test.
Results
Identification of compounds that inhibit intracellular growth and replication of Brucella abortus
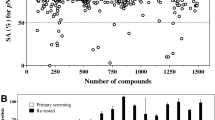
We sought to identify compounds from the ICCB library of 480 “known bioactives” that target host and/or pathogen to inhibit B. abortus infection. This library contains small-molecules whose mechanisms of actions have been established at a molecular level10. To quantify growth of B. abortus in the intracellular niche, we used a B. abortus strain7 that constitutively expresses mCherry fluorescent protein from a Tn7 transposon integrated in single copy at the chromosomal Tn7 attachment site (glmS)11. We screened for compounds that prevent entry (i.e., are effective when host cells are treated before infection, but not after infection) or intracellular replication. Specifically, we treated PMA-differentiated THP-1 macrophage-like cells with compounds from the ICCB library either 2 hours prior to infection or 4 hours after infection. The infected cells were incubated for an additional 72 hours prior to measuring fluorescent signal from intracellular B. abortus-mCherry. Using an MTT assay12, we assessed compound cytotoxicity to THP-1 cells at 72 hours (Supplementary Table S1). Compounds that decreased intracellular Brucella fluorescence by 20 percent or more in an initial bulk fluorescence screen were considered hits. This screen yielded 45 compounds that inhibited intracellular B. abortus growth when applied post infection, 20 of which were non-cytotoxic (Fig. 1). The pre-infection screen yielded 18 compounds that inhibited intracellular growth, seven of which were non-cytotoxic (Fig. 1). Six of the seven non-cytotoxic compounds identified in the pre-infection screen were among the 20 that inhibited growth when applied after infection (Fig. 1B). A single compound, EHNA, was effective only when applied prior to infection, suggesting that it may prevent Brucella entry into the host cell. In total, we identified 21 non-cytotoxic compounds that inhibit B. abortus infection/intracellular replication based on a bulk fluorescence assay (Supplementary Table S2). Validation of the hits identified using this fluorescence-based screen by two additional methods, enumeration of CFU and microscopy, is described below.
Identification of compounds that inhibit intracellular growth of B. abortus in a bulk human cell infection assay.
Compounds from the ICCB bioactives library were arrayed into plates containing THP-1 macrophage-like cells infected with wild-type B. abortus constitutively expressing mCherry. ICCB compounds are plotted sequentially along the X-axis; relative mCherry B. abortus fluorescence is plotted on the Y-axis in all graphs. Dotted line delineates 20% below the mean normalized fluorescence across all conditions; compounds that fall below this line were considered hits (orange). (A) Graph of B. abortus fluorescence (72 hours post-infection) in the presence of each ICCB compound in cells treated pre-infection (circles) or post-infection (squares) as described in Methods. (B) Twenty-one non-cytotoxic compounds that inhibit intracellular growth of B. abortus presumably by targeting the host. Orange-filled circles represent compounds that inhibited B. abortus infection by 20% or more. Green-filled circle represents a single compound that in our screen was found to be effective only when applied pre-infection.
Identification of compounds that inhibit metabolic activity of Brucella abortus
We next sought to identify compounds that directly inhibited B. abortus metabolism in axenic culture. To accomplish this, we adopted a modified phenotype microarray platform to screen the ICCB library for compounds that decrease metabolic activity of B. abortus cells grown in the presence of each compound over a period of 2 days. We monitored the metabolic activity of B. abortus by measuring reduction of tetrazolium dye spectrophotometrically: tetrazolium is reduced by metabolically active cells, eliciting a color change. This screen identified molecules that inhibited metabolic activity in a defined liquid medium growth platform13. Of the 480 ICCB compounds, we identified 26 that decrease B. abortus metabolic activity by ≥1 standard deviation from the mean (Fig. 2, Table 1). Three of these 26 Brucella-targeting compounds (Table 1) overlapped with the 21 compounds that inhibit intracellular B. abortus (Supplementary Table S2). We thus refer to the remaining 18 of the 21 compounds that inhibit intracellular Brucella infection/replication as host-specific compounds. We note that the 26 Brucella-targeting compounds reported here are not clinical antibiotics. The molecular targets of these compounds have been established in eukaryotic cells in some cases, though mechanisms of action may differ against bacteria.
Screen for molecules that inhibit Brucella abortus metabolism in vitro.
(A) A panel of six 96-well plates arrayed with 480 small molecules from the ICCB bioactives library. B. abortus growth/metabolism was assessed colorimetrically in test and control wells containing a minimal-defined base medium and tetrazolium dye as a metabolic indicator (purple color, monitored at 630 nm) as described in Methods. (B) B. abortus metabolism in minimal-defined medium in the presence of 480 ICCB compounds, as assessed by quantifying tetrazolium formazan absorbance at 630 nm. Orange circles represent twenty-six compounds that inhibited Brucella metabolism by ≥1 standard deviation from the mean (dotted horizontal line represents 1 standard deviation below the mean). ICCB compounds are plotted sequentially along the x-axis. The twenty-six hit compounds are listed in Table 1.
Compounds with activities against host and pathogen potently inhibit intracellular replication of B. abortus
We next sought to validate the cytotoxicity and inhibitory efficacy of hits presented above using additional experimental approaches. As described above, compound cytotoxicity to THP-1 cells was initially assessed using the MTT assay, which measures mitochondrial function. To avoid identification of false-positive cytotoxic compounds, we assayed cytotoxicity using two additional approaches. Specifically, we tested whether the 26 pathogen-targeting compounds (Table 1) and 18 host-specific compounds (Supplementary Table S2) were cytotoxic to THP-1 cells infected with B. abortus by imaging infected cells by fluorescence microscopy, (i) assessing defects in host cell morphology, and (ii) quantifying the average number of host nuclei per imaged field upon drug treatment (Fig. 3). Both of these are established methods to assess cell viability14,15. Of the 44 total compounds, 13 were toxic to human cells infected with B. abortus based on these imaging assays. All 13 of the cytotoxic compounds were identified as inhibitors of B. abortus metabolism in axenic culture (Table 1). Toxicity of 12 of the 13 compounds was consistent with the MTT cytotoxicity assay (Supplementary Table S1), which was measured on uninfected THP-1 cells. We removed these 13 compounds from further analysis. We retained seven compounds that were moderately cytotoxic based on quantification of host nuclei, but which did not result in noticeable defects in host cell morphology (Supplementary Fig. S1, Supplementary Table S3).
Microscopy-based assessment of hit compound cytotoxicity in Brucella-infected THP-1 cells.
Cytotoxicity of the 26 Brucella-specific (white bars) and 18 host-specific compounds (shaded bars) was assessed by quantifying the average number of nuclei in a viewing area per each treatment. The results are expressed as an average number of nuclei calculated from 6–24 images per treatment in a single experiment. Error bars represent standard deviation. Toxic compounds were also identified based on a qualitative image analysis. Compounds that had a significantly lower average number of nuclei than control (p < 0.05) were deemed toxic. While compounds that are labeled as moderately-toxic had a lower average number of nuclei, the image analysis revealed adherent cells that had normal morphology (Supplementary Fig. S1). Compounds that had no apparent affect on morphology and had a high average number of nuclei were deemed non-toxic. *Three overlapping host- and Brucella-specific compounds.
To test whether compounds identified as inhibitors of B. abortus metabolism in axenic culture also inhibited replication in the intracellular niche, we imaged infected THP-1 cells and quantified fluorescent brucellae. Specifically, we calculated the average bacteria-to-host nucleus ratio in the presence of each of the 13 Brucella-targeting compounds that were deemed either non-cytotoxic or moderately cytotoxic to THP-1 (Fig. 3, Supplementary Table S3). This imaging approach identified five compounds that significantly inhibited intracellular replication of B. abortus (Fig. 4A,B). As further validation, we lysed THP-1 cells treated with this same set of hit compounds and plated for B. abortus CFUs 3 days post-infection. Enumeration of B. abortus CFUs from infected THP-1 cells confirmed the efficacy of 3 out of the 5 compounds as inhibitors of B. abortus replication and survival in the intracellular niche; treatment of THP-1 with 2-APB showed significant reduction of CFU, but no significant difference in our imaging assay (Fig. 4C). Only three hits, tyrphostin 9, carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP), and mitomycin C, were significantly effective (p-value < 0.05) across all three functional assays. N-phenylanthranilic acid, niflumic acid, NPPB, 2-APB, H9, methotrexate, flufenamic acid, AG-879, manumycin A, and TPCK did not inhibit B. abortus infection in all three assays at the tested concentrations but, given different sensitivities of each assay and the ability of these compounds to inhibit B. abortus metabolism in axenic culture, these molecules still represent candidate inhibitors of Brucella in the intracellular niche. Our final list of hits includes 13 compounds that were initially identified as metabolic inhibitors. Of these 13 hits, three robustly inhibited intracellular B. abortus replication across all three assays, three compounds were effective in two assays. Seven hit compounds inhibited only in the initial screen (Table 2).
Efficacy of non-cytotoxic pathogen-targeting compounds as inhibitors of B. abortus intracellular replication in a THP-1 infection model.
(A) Quantification of inhibitory efficacy of compounds, measuring the average ratio of bacteria-to-nucleus in THP-1 cells treated with each of the 13 non-cytotoxic pathogen-targeting compounds in a single experiment. Statistical significance was evaluated from ≈7 images per treatment using one-way ANOVA followed by Dunnett’s test (*p < 0.05, ***p < 0.001, ****p < 0.0001). Error bars represent +/− standard deviation. (B) Sample fluorescence images of nuclei (blue) and intracellular B. abortus (pseudocolor green) assessing the efficacy of each of the 5 final pathogen-targeting compounds that significantly inhibited infection by B. abortus: tyrphostin 9, FCCP, AG-879, manumycin A, and mitomycin C. Doxycycline is a positive control and TPCK represents a non-hit compound that did not have any effect on infection. Scale bar = 50 μm. (C) B. abortus CFU counts generated from infected THP-1 treated with 13 identified non-toxic compounds that inhibit B. abortus metabolism in axenic culture. Data represent an average from 3–7 biological replicates. Error bars represent +/− SEM.
We applied the same image analysis and CFU-enumeration pipeline to test the efficacy of the 18 non-cytotoxic host-specific compounds (Fig. 3) that had no effect on B. abortus metabolism in axenic culture (Supplementary Table S3). This approach provided us with independent efficacy measurements of compounds that inhibit replication of intracellular Brucella in the initial bulk fluorescence assay. Imaging showed that 9 of 18 non-cytotoxic compounds identified in the bulk fluorescence screen significantly inhibited replication of intracellular bacteria (Fig. 5A,B, Table 2). The CFU assay confirmed a single hit, nicardipine, which was also effective in two previous functional assays (Fig. 5C). Hence, one of the 18 non-cytotoxic host-specific compounds showed efficacy across all three inhibition assays and nine were effective in two assays. The remaining eight hits showed efficacy only in the initial screen (Table 2).
Efficacy of non-cytotoxic host-specific compounds as inhibitors of B. abortus intracellular replication in a THP-1 infection model.
(A) Quantification of inhibitory efficacy of compounds, measuring the average ratio of bacteria-to-nucleus in THP-1 cells treated with each of the 18 non-cytotoxic host-specific compounds in a single experiment. Statistical significance was evaluated from ≈7 images per treatment using one-way ANOVA followed by Dunnett’s test (*p < 0.05, ****p < 0.0001). Error bars represent +/− standard deviation. (B) Sample fluorescence images of nuclei (blue) and intracellular B. abortus (pseudocolor green) assessing efficacy of each of the 9 final host-specific compounds that significantly inhibited infection by B. abortus. Doxycycline is a positive control and AG-213 represents a non-hit compound that did not have any effect on infection. Scale bar = 50 μm. (C) B. abortus CFU counts generated from infected THP-1 treated with 18 identified non-toxic compounds that presumably target host cells to inhibit B. abortus infection. Data represent an average from 3–7 biological replicates. Error bars represent +/− SEM.
Each of the functional assays used to identify anti-infective activity of compounds offers different sensitivity. To more closely examine inhibitory efficacy, we determined the minimum inhibitory concentrations (MICs) of selected compounds that were effective across at least two functional assays; these included tryphostin 9, AG-879, nicardipine, FCCP, mitomycin C, arvanil, W-7, 2-APB, and 1400W. The MICs were determined based on the lowest compound concentration that significantly (p < 0.05) decreased count of intracellular bacteria as assessed by fluorescence microscopy (Supplementary Fig. S4). While the MICs for seven compounds were lower than the initial screen concentration, two compounds, 2-APB and 1400W, did not show significant inhibition at concentrations lower than the initial screen (Table 3; Fig. 6). These results are consistent with our initial imaging data presented in Table 2 and Figs 4 and 5. The MIC measurements support the conclusion that these compounds are inhibitors of B. abortus in the intracellular niche
Inhibition of B. abortus replication in THP-1 cells at MIC.
Images of B. abortus (green pseudocolor) and Hoechst-stained THP-1 nuclei (blue) of cells treated with compunds at their respective minimum inhibitory concentrations (MICs). The images were taken 72 hours post-infection. Control column represents mock treated controls matching each treatment in the Compound column. Magnified inset regions (white box) appear to the right of each image. Scale bars = 50 μm.
In summary, we have identified 4 robust B. abortus antimicrobials, one of which (nicardipine) inhibits replication specifically in the host niche but not in axenic culture. Three compounds: tyrphostin 9, FCCP, and mitomycin C, have well-established molecular targets in the host-cell, but also inhibit Brucella metabolism and its intracellular replication (Table 2). The latter category of compounds, which have activities against host and pathogen, are the most potent inhibitors in our screen (Figs 4C and 5C, Tables 2 and 3). These compounds represent a potentially novel class of Brucella anti-infectives; their efficacy may arise from their ability to coordinately perturb host and pathogen. The remaining host- and pathogen-targeting compounds (Table 2) represent promising anti-infective hits that may be developed for combination therapeutic approaches that simultaneously target pathogen and host.
Discussion
Activities and Targets of Compounds that inhibit Brucella metabolism in axenic culture
We identified 26 compounds that directly target B. abortus to inhibit its metabolic activity (Table 1). The largest class of inhibitory compounds is kinase inhibitors. While the mechanism(s) by which these compounds inhibit B. abortus respiration remain undefined, previous reports have demonstrated that eukaryotic kinase inhibitors have antibacterial activity7,16,17. Of these 26 compounds, 5 exhibit significant structural similarity: flufenamic acid, 5-Nitro-2-(3-phenylpropylaminol)benzoic acid (NPPB), n-phenylanthranilic acid, niflumic acid, and LY-83583 (Supplementary Fig. S2A). All, with the exception of LY-83583, are known to target ion homeostasis, which is the second largest class of compounds that inhibit B. abortus metabolism in axenic culture (Table 1). Two naphthoquinones, juglone and beta-lapachone, also exhibit significant structural similarity (Supplementary Fig. S2A). Although each of these compounds are potent modulators of eukaryotic cell biology, both have reported antibacterial activity18,19 suggesting they may coordinately perturb host and pathogen. Four additional classes of hit compounds include metal chelators, inhibitors of energy metabolism, DNA modifiers, and inhibitors of lipid biosynthesis. The identification of metal chelators as inhibitors is not surprising given that a number of metals, including zinc, iron, manganese, and magnesium are essential for B. abortus growth in in vivo and in vitro models20,21. Nonetheless, they provide attractive therapeutic candidates.
Three Brucella-targeting compounds, FCCP, diphenyleneiodonium, and methotrexate, are known to target energy metabolism and may therefore intercept Brucella metabolic pathways and decrease the cellular pool of reducing equivalents. Among these three compounds, methotrexate, is an FDA-approved antimetabolite drug known for inhibiting dihydrofolate reductase (DHFR). Interestingly, FDA-approved DHFR inhibitors are used as antiprotozoal and antimicrobials22. FCCP, a known uncoupling ionophore was previously reported to inhibit intracellular transport of secretory viral glycoproteins at concentrations overlapping with the MIC identified in our study23. Two DNA-targeting agents, mitomycin C and beta-lapachone, are also established anti-infectives24,25. Both cerulenin and cinnamyl-3,4-dihydroxy-α-cyanocinnamate (CDC) are known inhibitors of lipid26,27 and sterol biogenesis28 in eukaryotes, but also exhibit a direct antimicrobial activity29,30. Sterols have been reported as important host factors in macrophage infection by B. abortus31. CDC, in particular, is notable as it inhibits 12- and 5-lipoxygenase27, which have specific host activities that are required for Streptococcus pneumoniae lung infection and B. abortus infection in mice32,33. Cerulenin and CDC thus have the potential to target both the host and the pathogen in certain infections. The remaining five compounds fall into the “Other” class, these include: NSC-95397, LY-83583, wiskostatin, nigericin, and tosyl-phe-CMK (TPCK). Previous studies report antibacterial activities of wiskostatin and nigericin34,35. Collectively, many of these 26 compounds represent novel metabolic inhibitors that effectively target Brucella and perhaps other bacteria.
Pharmacological Features of Brucella-targeting compounds that inhibit replication in the intracellular niche
Though each of the 26 identified compounds shown in Table 1 potently inhibit bacterial metabolism, many are cytotoxic to human cells at our screen concentrations. Thirteen of the 26 Brucella-targeting compounds were classified as non-toxic or moderately toxic to THP-1 cells based on our assays (Fig. 3). Of the 13 compounds, tyrphostin 9, FCCP, and mitomycin C, were robust inhibitors of B. abortus replication in the intracellular niche across all three inhibition assays (Table 2). An additional three Brucella-targeting compounds, AG-879, manumycin A, and 2-APB, were effective in two of the three intracellular replication assays. The remaining seven hits effectively inhibited intracellular replication as measured the initial fluorescence screen, but inhibition was not observed by fluorescence imaging and enumeration of CFUs (Table 2).
Many non-cytotoxic compounds that inhibit B. abortus in pure culture have well-documented ability to perturb eukaryotic cellular processes. Notably, a number of compounds that inhibit B. abortus in axenic medium, including tyrphostin 9, AG-879, FCCP, manumycin A, and mitomycin C, are known to affect mitochondrial function either by targeting the oxidative phosphorylation or mitochondrial membrane potential36,37,38,39. In addition to overlapping function in targeting mitochondria, tyrphostin 9 and AG-879 have almost identical structures and have also been reported to inhibit protein kinases (Supplementary Fig. S3)40,41. Host-kinase signaling has been implicated in a number of viral and bacterial infections, including infection by B. abortus42,43,44,45,46. FCCP is also reported to disrupt host microtubules, which is essential for Brucella infection46,47. Manumycin A, a compound that inhibits B. abortus metabolism, is a known inhibitor of farnesyltransferase activity in eukaryotic cells48, and has been reported to inhibit Anaplasma phagocytophilum by directly targeting the pathogen, but not the host49.
Although, it is not known whether these compounds inhibit B. abortus in the intracellular niche by targeting the pathogen only, or by targeting both the host and B. abortus, we note interesting congruence between our final hit list with compounds that target host pathways to inhibit Type IV effector protein translocation by Legionella pneumophila50. Tyrphostin 9, W-7, caffeic acid phenethyl ester (CAPE), FCCP, and HA14-1 are among the overlapping Legionella/Brucella hits, and Type IV secretion is a crucial requirement of B. abortus virulence in cell-based and animal infection models51,52. The coordinate effects of these compounds on host and microbial energy metabolism and host cell pathways may contribute to their efficacy as inhibitors of intracellular B. abortus replication.
Activities and Targets of Host-specific compounds that inhibit replication of B. abortus in the intracellular niche
Eighteen of the thirty-one compounds that inhibited replication of intracellular B. abortus had no direct effect on Brucella metabolism in axenic culture. Therefore, these are candidate compounds that inhibit intracellular B. abortus replication by targeting the host, but not the pathogen. The inhibitory efficacy of a single compound, nicardipine, was consistent across all three assays (fluorescence, cell imaging, and CFU counts), nine compounds were effective across two assays, and the remaining eight inhibited infection only in the initial fluorescence screen (Table 2).
Two of the five host-specific compounds that target host kinase signaling pathways effectively inhibited infection across two functional assays. These include A-3 and W-7, which is a calmodulin antagonist (Table 2)53. A-3 and W-7 are structurally similar (Supplementary Fig. S2B, Supplementary Fig. S3), suggesting that they may target the same host pathway to interfere with intracellular Brucella growth and replication; the cAMP/cGMP-dependent protein kinases, PKA and PKG, are common targets of these compounds54,55. Erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) is an inhibitor of phosphodiesterase-2 (PDE2) that hydrolyzes cAMP and cGMP, which are responsible for activation of PKA and PKG, respectively56,57. Among all host-targeting compounds, two pairs showed significant structure similarity: ion channel ligands (nicardipine and NPPB) and protease inhibitors (MDL-28170 and Z-prolyl-prolinal) (Supplementary Fig. S2B). Structural similarity between these compounds suggests shared anti-infective mechanisms of action, though follow-up experiments focused on mechanism of action are required.
Four additional hits, W-7, nicardipine, arvanil, and kavain, are known to affect calcium homeostasis. W-7 was previously reported to have antibacterial activity against a number of intracellular bacterial pathogens that can replicate inside host cells, including Ehrlichia and Pseudomonas58,59. Our previous work on host-targeted anti-infectives identified a number of calcium channel-targeting FDA-approved drugs that were effective in the inhibition of intracellular bacteria7. Nifedipine, a dihydropyridine calcium channel blocker that is structurally related to nicardipine, was shown to inhibit Brucella infection in bone marrow-derived macrophages. This result is congruent with the data reported herein, and provides evidence that these compounds will be effective in different cell types60. Together, these results further underscore the importance of host calcium homeostasis during Brucella intracellular infection and growth.
AA-861, HA14-1, and CAPE, had no effect on B. abortus growth and replication in axenic culture, but inhibited intracellular B. abortus replication across two functional assays. In agreement with our finding that AA-861, a 5-lipoxygenase (5-LO) inhibitor, attenuates intracellular growth and replication of Brucella, a recent study demonstrated that 5-LO deficient mice are resistant to infection by B. abortus33. Notably, CAPE, an NF-κB inhibitor, was also shown to inhibit 5-LO61. Identification of HA14-1, a pro-apoptotic Bcl-2 inhibitor, suggests that activating apoptosis may interfere with Brucella infection. Indeed, inhibition of apoptosis is reported to be beneficial to Brucella intracellular growth and survival62,63. Finally, of the two nitric oxide synthase (NOS) inhibitors, 1400W and L-NAME, only 1400W was effective across two growth inhibition assays. Identification of these inhibitors suggests that nitric oxide may be beneficial to the growth/replication of B. abortus in THP-1 macrophages. Infection of mammalian cells by Listeria monocytogenes relies on host-derived nitric oxide generated by NOS64. However, inhibition of murine NOS was shown to promote Brucella infection, but only under specific conditions (using IFN-γ-treated cells and opsonized-brucellae)65. The exact mechanism of action of each of the newly identified B. abortus inhibitors remains to be determined.
In summary, we identified molecules that inhibit B. abortus cellular metabolism and intracellular growth. Our data suggest that molecules targeting both pathogen and host can enhance inhibition of intracellular B. abortus replication and survival in a human macrophage infection model. Among the identified inhibitors of B. abortus in the intracellular niche, we note enrichment of broad-spectrum kinase inhibitors, compounds that target ion homeostasis, and compounds that target mitochondrial function. Compounds that target host and pathogen, either as a single “dual-target” molecules or in combination, may be developed for use against a broader spectrum of intracellular microbial infections, including eukaryotic parasites. Like other host-targeting molecules, dual-targeting compounds hold the potential of limiting the emergence of antibiotic resistance66. Future studies in animal models of infection and disease will test the antimicrobial efficacy of these compounds in vivo.
Additional Information
How to cite this article: Czyż, D. M. et al. A dual-targeting approach to inhibit Brucella abortus replication in human cells. Sci. Rep. 6, 35835; doi: 10.1038/srep35835 (2016).
References
Pappas, G., Papadimitriou, P., Akritidis, N., Christou, L. & Tsianos, E. V. The new global map of human brucellosis. Lancet Infect Dis 6, 91–99 (2006).
Pappas, G., Akritidis, N., Bosilkovski, M. & Tsianos, E. Brucellosis. N Engl J Med 352, 2325–2336, doi: 352/22/2325 [pii] 10.1056/NEJMra050570 (2005).
Dean, A. S., Crump, L., Greter, H., Schelling, E. & Zinsstag, J. Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl Trop Dis 6, e1865, doi: 10.1371/journal.pntd.0001865 (2012).
Moreno, E. & Moriyon, I. In The Prokaryotes-A Handbook on the Biology of Bacteria Vol. Volume 5: Proteobacteria: Alpha and Beta Subclasses (eds M. Dworkin et al.) 315–456 (2006).
Rabbani-Anari, M. et al. Antibiotics for treating human brucellosis. 1–7 (The Cochrane Collaboration, Oxford, UK., 2009).
Ariza, J. et al. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med 4, e317, doi: 10.1371/journal.pmed.0040317 (2007).
Czyz, D. M. et al. Host-directed antimicrobial drugs with broad-spectrum efficacy against intracellular bacterial pathogens. MBio 5, e01534–01514, doi: 10.1128/mBio.01534-14 (2014).
Bolton, E. E., Wang, Y., Thiessen, P. A. & Bryant, S. H. In Annual Reports in Computational Chemistry Vol. Volume 4 (eds. A. Wheeler Ralph & C. Spellmeyer David ) 217–241 (Elsevier, 2008).
Kim, S., Bolton, E. E. & Bryant, S. H. Effects of multiple conformers per compound upon 3-D similarity search and bioassay data analysis. Journal of Cheminformatics 4, 28–28, doi: 10.1186/1758-2946-4-28 (2012).
Shelat, A. A. & Guy, R. K. Scaffold composition and biological relevance of screening libraries. Nat Chem Biol 3, 442–446, doi: 10.1038/nchembio0807-442 (2007).
Choi, K. H. & Schweizer, H. P. mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat Protoc 1, 153–161, doi: 10.1038/nprot.2006.24 (2006).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65, 55–63, 10.1016/0022-1759(83)90303-4 (1983).
Bochner, B. R. Global phenotypic characterization of bacteria. FEMS Microbiol Rev 33, 191–205, doi: 10.1111/j.1574-6976.2008.00149.x (2009).
Ramirez, C. N., Antczak, C. & Djaballah, H. Cell Viability Assessment: Toward Content-Rich Platforms. Expert opinion on drug discovery 5, 223–233, doi: 10.1517/17460441003596685 (2010).
Johnson, S., Nguyen, V. & Coder, D. In Current Protocols in Cytometry (John Wiley & Sons, Inc., 2001).
Miller, J. R. et al. A class of selective antibacterials derived from a protein kinase inhibitor pharmacophore. Proc Natl Acad Sci USA 106, 1737–1742, doi: 10.1073/pnas.0811275106 (2009).
Walsh, C. T. & Fischbach, M. A. Repurposing libraries of eukaryotic protein kinase inhibitors for antibiotic discovery. Proc Natl Acad Sci USA 106, 1689–1690, doi: 10.1073/pnas.0813405106 (2009).
Macedo, L. et al. beta-Lapachone activity in synergy with conventional antimicrobials against methicillin resistant Staphylococcus aureus strains. Phytomedicine 21, 25–29, doi: 10.1016/j.phymed.2013.08.010 (2013).
Clark, A. M., Jurgens, T. M. & Hufford, C. D. Antimicrobial activity of juglone. Phytotherapy Research 4, 11–14, doi: 10.1002/ptr.2650040104 (1990).
Roop, R. M., 2nd . Metal acquisition and virulence in Brucella. Anim Health Res Rev 13, 10–20, doi: 10.1017/S1466252312000047 (2012).
Sheehan, L. M., Budnick, J. A., Roop, R. M. & Caswell, C. C. Coordinated Zinc Homeostasis Is Essential for the Wild-Type Virulence of Brucella abortus. Journal of Bacteriology 197, 1582–1591, doi: 10.1128/JB.02543-14 (2015).
Wright, D. L. & Anderson, A. C. Antifolate Agents: A Patent Review (2006–2010). Expert opinion on therapeutic patents 21, 1293–1308, doi: 10.1517/13543776.2011.587804 (2011).
Kaariainen, L., Hashimoto, K., Saraste, J., Virtanen, I. & Penttinen, K. Monensin and FCCP inhibit the intracellular transport of alphavirus membrane glycoproteins. J Cell Biol 87, 783–791 (1980).
Shiba, S., Terawaki, A., Taguchi, T. & Kawamata, J. Selective inhibition of formation of deoxyribonucleic acid in Escherichia coli by mitomycin C. Nature 183, 1056–1057 (1959).
Cruz, F. S., Docampo, R. & Boveris, A. Generation of superoxide anions and hydrogen peroxide from beta-lapachone in bacteria. Antimicrob Agents Chemother 14, 630–633 (1978).
D’Agnolo, G., Rosenfeld, I. S., Awaya, J., Omura, S. & Vagelos, P. R. Inhibition of fatty acid synthesis by the antibiotic cerulenin. Specific inactivation of beta-ketoacyl-acyl carrier protein synthetase. Biochim Biophys Acta 326, 155–156 (1973).
Pergola, C. et al. Cinnamyl-3,4-dihydroxy-alpha-cyanocinnamate is a potent inhibitor of 5-lipoxygenase. J Pharmacol Exp Ther 338, 205–213, doi: 10.1124/jpet.111.180794 (2011).
Ohno, T., Awaya, J., Kesado, T., Nomura, S. & Omura, S. Mechanism of action of CM-55, a synthetic analogue of the antilipogenic antibiotic cerulenin. Antimicrob Agents Chemother 6, 387–392 (1974).
Wille, W., Eisenstadt, E. & Willecke, K. Inhibition of De Novo Fatty Acid Synthesis by the Antibiotic Cerulenin in Bacillus subtilis: Effects on Citrate-Mg(2+) Transport and Synthesis of Macromolecules. Antimicrob Agents Chemother 8, 231–237 (1975).
Heath, R. J. & Rock, C. O. Fatty acid biosynthesis as a target for novel antibacterials. Current opinion in investigational drugs (London, England, : 2000) 5, 146–153 (2004).
Watarai, M. et al. Macrophage plasma membrane cholesterol contributes to Brucella abortus infection of mice. Infect Immun 70, 4818–4825 (2002).
Bhowmick, R. et al. Systemic disease during Streptococcus pneumoniae acute lung infection requires 12-lipoxygenase-dependent inflammation. J Immunol 191, 5115–5123, doi: 10.4049/jimmunol.1300522 (2013).
Fahel, J. S. et al. 5-Lipoxygenase Negatively Regulates Th1 Response during Brucella abortus Infection in Mice. Infection and Immunity 83, 1210–1216, doi: 10.1128/iai.02592-14 (2015).
Brown, D. R. & Price, L. D. Characterization of Salmonella enterica serovar Typhimurium DT104 invasion in an epithelial cell line (IPEC J2) from porcine small intestine. Vet Microbiol 120, 328–333, doi: 10.1016/j.vetmic.2006.11.001 (2007).
Graven, S. N., Estrada-O, S. & Lardy, H. A. Alkali metal cation release and respiratory inhibition induced by nigericin in rat liver mitochondria. Proc Natl Acad Sci USA 56, 654–658 (1966).
Sakamuru, S. et al. Application of a homogenous membrane potential assay to assess mitochondrial function. Physiological Genomics 44, 495–503, doi: 10.1152/physiolgenomics.00161.2011 (2012).
Heytler, P. G. Uncouplers of oxidation phosphorylation. Pharmacology & Therapeutics 10, 461–472, 10.1016/0163-7258(80)90027-3 (1980).
Ali, B. R., Pal, A., Croft, S. L., Taylor, R. J. & Field, M. C. The farnesyltransferase inhibitor manumycin A is a novel trypanocide with a complex mode of action including major effects on mitochondria. Molecular and biochemical parasitology 104, 67–80, doi: 10.1016/s0166-6851(99)00131-0 (1999).
Seong, G. J. et al. Mitomycin-C Induces the Apoptosis of Human Tenon’s Capsule Fibroblast by Activation of c-Jun N-Terminal Kinase 1 and Caspase-3 Protease. Investigative Ophthalmology & Visual Science 46, 3545–3552, doi: 10.1167/iovs.04-1358 (2005).
Levitzki, A. & Gilon, C. Tyrphostins as molecular tools and potential antiproliferative drugs. Trends in Pharmacological Sciences 12, 171–174, doi: 10.1016/0165-6147(91)90538-4.
He, H. et al. The Tyr-kinase inhibitor AG879, that blocks the ETK-PAK1 interaction, suppresses the RAS-induced PAK1 activation and malignant transformation. Cancer Biol Ther 3, 96–101 (2004).
Kumar, N., Sharma, N. R., Ly, H., Parslow, T. G. & Liang, Y. Receptor Tyrosine Kinase Inhibitors That Block Replication of Influenza A and Other Viruses. Antimicrob Agents Chemother 55, 5553–5559, doi: 10.1128/aac.00725-11 (2011).
Kumar, N., Liang, Y., Parslow, T. G. & Liang, Y. Receptor Tyrosine Kinase Inhibitors Block Multiple Steps of Influenza A Virus Replication. Journal of Virology 85, 2818–2827, doi: 10.1128/JVI.01969-10 (2011).
Burton, E. A., Plattner, R. & Pendergast, A. M. Abl tyrosine kinases are required for infection by Shigella flexneri. The EMBO Journal 22, 5471–5479, doi: 10.1093/emboj/cdg512 (2003).
Krachler, A. M., Woolery, A. R. & Orth, K. Manipulation of kinase signaling by bacterial pathogens. J Cell Biol 195, 1083–1092, doi: 10.1083/jcb.201107132 (2011).
Guzmán-Verri, C. et al. GTPases of the Rho Subfamily Are Required for Brucella abortus Internalization in Nonprofessional Phagocytes: DIRECT ACTIVATION OF Cdc42. Journal of Biological Chemistry 276, 44435–44443, doi: 10.1074/jbc.M105606200 (2001).
Maro, B., Marty, M. C. & Bornens, M. In vivo and in vitro effects of the mitochondrial uncoupler FCCP on microtubules. The EMBO Journal 1, 1347–1352 (1982).
Hara, M. et al. Identification of Ras farnesyltransferase inhibitors by microbial screening. Proc Natl Acad Sci USA 90, 2281–2285 (1993).
Xiong, Q. & Rikihisa, Y. The prenylation inhibitor manumycin A reduces the viability of Anaplasma phagocytophilum. J Med Microbiol 60, 744–749, doi: 10.1099/jmm.0.029231-0 (2011).
Charpentier, X. et al. Chemical Genetics Reveals Bacterial and Host Cell Functions Critical for Type IV Effector Translocation by Legionella pneumophila. PLoS Pathogens 5, e1000501, doi: 10.1371/journal.ppat.1000501 (2009).
Hong, P. C., Tsolis, R. M. & Ficht, T. A. Identification of Genes Required for Chronic Persistence of Brucella abortus in Mice. Infection and Immunity 68, 4102–4107, doi: 10.1128/iai.68.7.4102-4107.2000 (2000).
Sieira, R., Comerci, D. J., Sánchez, D. O. & Ugalde, R. A. A Homologue of an Operon Required for DNA Transfer in Agrobacterium Is Required in Brucella abortus for Virulence and Intracellular Multiplication. Journal of Bacteriology 182, 4849–4855 (2000).
Inagaki, M. et al. Naphthalenesulfonamides as calmodulin antagonists and protein kinase inhibitors. Molecular Pharmacology 29, 577–581 (1986).
Hidaka, H. & Kobayashi, R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol 32, 377–397, doi: 10.1146/annurev.pa.32.040192.002113 (1992).
Hidaka, H., Inagaki, M., Kawamoto, S. & Sasaki, Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide-dependent protein kinase and protein kinase C. Biochemistry 23, 5036–5041, doi: 10.1021/bi00316a032 (1984).
Taylor, S. S., Buechler, J. A. & Yonemoto, W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem 59, 971–1005, doi: 10.1146/annurev.bi.59.070190.004543 (1990).
Omori, K. & Kotera, J. Overview of PDEs and Their Regulation. Circulation Research 100, 309–327, doi: 10.1161/01.RES.0000256354.95791.f1 (2007).
Rikihisa, Y., Zhang, Y. & Park, J. Role of Ca2+ and calmodulin in ehrlichial infection in macrophages. Infection and Immunity 63, 2310–2316 (1995).
Evans, D. J., Frank, D. W., Finck-Barbançon, V., Wu, C. & Fleiszig, S. M. J. Pseudomonas aeruginosa Invasion and Cytotoxicity Are Independent Events, Both of Which Involve Protein Tyrosine Kinase Activity. Infection and Immunity 66, 1453–1459 (1998).
Kim, D. H. et al. RGS2-mediated intracellular Ca2+ level plays a key role in the intracellular replication of Brucella abortus within phagocytes. J Infect Dis 205, 445–452, doi: 10.1093/infdis/jir765 (2012).
Sud’ina, G. F. et al. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Letters 329, 21–24, doi: 10.1016/0014-5793(93)80184-V (1993).
Wei, P. et al. A20 promotes Brucella intracellular growth via inhibition of macrophage cell death and activation. Vet Microbiol 175, 50–57, 10.1016/j.vetmic.2014.11.006 (2015).
Gross, A., Terraza, A., Ouahrani-Bettache, S., Liautard, J.-P. & Dornand, J. In Vitro Brucella suis Infection Prevents the Programmed Cell Death of Human Monocytic Cells. Infection and Immunity 68, 342–351 (2000).
Cole, C., Thomas, S., Filak, H., Henson, Peter M. & Lenz, Laurel L. Nitric Oxide Increases Susceptibility of Toll-like Receptor-Activated Macrophages to Spreading Listeria monocytogenes. Immunity 36, 807–820, 10.1016/j.immuni.2012.03.011 (2012).
Gross, A. et al. Expression and Bactericidal Activity of Nitric Oxide Synthase in Brucella suis-Infected Murine Macrophages. Infection and Immunity 66, 1309–1316 (1998).
Lebeis, S. L. & Kalman, D. Aligning antimicrobial drug discovery with complex and redundant host-pathogen interactions. Cell Host Microbe 5, 114–122, doi: 10.1016/j.chom.2009.01.008 (2009).
Acknowledgements
We acknowledge the support staff of the Howard Taylor Ricketts Regional Biocontainment Laboratory, and Aretha Fiebig for assistance with figure design. This study was funded in part by NIH grants 1U19AI107792 and 1R01AI107159 to S.C. and the Chicago Biomedical Consortium with support from the Searle Funds at the Chicago Community Trust to D.M.C.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: D.M.C., N.J.-G., H.A.S. and S.C. Performed the experiments: D.M.C. and N.J.-G. Analyzed the data: D.M.C. and S.C. Contributed reagents/materials/analysis tools: H.A.S. Wrote the paper: D.M.C. and S.C. All authors reviewed and approved the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Czyż, D., Jain-Gupta, N., Shuman, H. et al. A dual-targeting approach to inhibit Brucella abortus replication in human cells. Sci Rep 6, 35835 (2016). https://doi.org/10.1038/srep35835
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35835
This article is cited by
-
Lubeluzole: from anti-ischemic drug to preclinical antidiarrheal studies
Pharmacological Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.