Abstract
Internal browning (IB), a physiological disorder (PD) that causes severe losses in harvested pineapple, can be induced by exogenous gibberellins (GAs). Over the years, studies have focused on roles of Gibberellin 2-oxidase (GA2oxs), the major GAs catabolic enzyme in plants, in the regulation of changes in morphology or biomass. However, whether GA2oxs could regulate PD has not been reported. Here, a full-length AcGA2ox cDNA was isolated from pineapple, with the putative protein sharing 23.59% to 72.92% identity with GA2oxs from five other plants. Pineapples stored at 5 °C stayed intact, while those stored at 20 °C showed severe IB. Storage at 5 °C enhanced AcGA2ox expression and decreased levels of a GAs (GA4) ‘compared with storage at 20 °C. However, at 20 °C, exogenous application of abscisic acid (ABA) significantly suppressed IB. ABA simultaneously upregulated AcGA2ox and reduced GA4. Ectopic expression of AcGA2ox in Arabidopsis resulted in reduced GA4, lower seed germination, and shorter hypocotyls and roots, all of which were restored by exogenous GA4/7. Moreover, in pineapple, GA4/7 upregulated polyphenol oxidase, while storage at 5 °C and ABA downregulated it. These results strongly suggest the involvement of AcGA2ox in regulation of GAs levels and a role of AcGA2ox in regulating IB.
Similar content being viewed by others
Introduction
Internal browning (IB), also referred to as blackheart, is a physiological disorder (PD) of pineapple (Ananas comosus [L.] Merr.) commonly occurring during storage and shipment, which results in severe loss of commercial value1. Exogenous gibberellins (GAs) treatment induces IB1,2,3, while low temperature storage inhibits it2,4,5. Low temperature (4 °C) increases the level of bioactive GAs in Arabidopsis seeds6 but reduces GAs levels and inhibits growth of Arabidopsis seedlings7. How low temperature influences GAs biosynthesis in harvested pineapple remains unclear.
GAs are endogenous phytohormones that are involved in the regulation of the life cycle of plants, including seed germination, leaf expansion, stem elongation, floral induction and fruit maturation8. The major bioactive GAs, such as GA1 and GA4, are synthesized from trans-geranylgeranyl diphosphate by the sequential actions of cyclases, membrane-associated mono-oxygenases and soluble 2-oxoglutarate-dependent dioxygenases9. The dioxygenases catalyze the later steps of GA biosynthesis, including the removal of the C-20 group and the introduction of the 3β-hydroxyl group. Negative feedback is a central mechanism of GAs biosynthesis, such as the GA 20-oxidase genes and a GA 3β-hydroxylase gene of Arabidopsis, both of whose transcript levels are subject to feedback regulation10, indicating their roles in the maintenance of the endogenous levels of bioactive GAs11.
The GAs catabolism is another important factor that regulates the endogenous levels of bioactive GAs11. In many plant species, bioactive GAs and their immediate precursors are 2β-hydroxylated by GA 2-oxidase (GA2ox) to produce biologically inactive products12. Genes encoding GA2ox s were first identified in 199913,14,15. Over the years, the functions of GA2oxs have been well-documented. Overexpression of GA2ox genes caused decreases in levels of bioactive GAs and dwarfing phenotypes in rice11, poplar16, Arabidopsis17 and wheat18. In fact, by limiting bioactive GA content, they regulate development at various stages during the plant life cycle. They prevent seed germination in the absence of light and cold stimuli, delay the vegetative and floral phase transitions, limit the number of flowers produced per inflorescence, suppress elongation of the pistil prior to fertilization19, influence underground biomass growth of Populus20, and affect fruit set and growth in tomato21. Our recent work suggested that downregulation of a GA2ox could be related to the delay of senescence of a harvested leafy vegetable22. However, to the authors’ knowledge, there has not been any study on the role GA2oxs in regulating PD. IB is one of the most typical and devastating PDs of pineapple whose occurrence is related to application of GAs1,2,3. Therefore, IB is an ideal PD for addressing the role of GA2oxs. Studying AcGA2ox gene in the context of IB would surely add to the understanding of the functions of GA2oxs gene family.
Here through displaying the expression patterns of AcGA2ox gene in response to a series of treatments that regulate GA levels and IB development in pineapple, in combination with the phenotypes connected with deficiency in bioactive GAs of Arabidopsis lines overexpressing AcGA2ox, we show that enhanced expression of AcGA2ox resulted in lower bioactive GA levels and thus suppressed IB development.
Results
Cloning and Characterization of AcGA2ox cDNA
By means of homologous cloning and RACE-PCR, the full-length AcGA2ox cDNA of 1367 bp was isolated, with an open reading frame consisting of 981 bp, encoding a protein of 326 amino acids and designated AcGA2ox (GenBank accession number FJ645911) (Fig. S1). Sequence analysis shows that the putative AcGA2ox protein, with molecular weight of 36.2 kD, and a pI of 5.95 according to Compute pI/MW program, contains amino acids that are conserved for GA2oxs, including His-113, Asp-115, and His-272 (Fig. S1) in the 2OG-FeII_Oxy domain. These residues are thought to associate at the catalytic site and bind with Fe2+ 23. In addition, the POSORT and MotifScan analyses show that AcGA2ox has six protein kinase C phosphorylation sites and six casein kinase II phosphorylation sites, similar to GA2oxs from other plants. Alignment of the putative pineapple AcGA2ox with all the known GA2oxs from Arabidopsis, rice, tomato, tobacco and oil palm showed identities between 23.59% and 72.92%%. AcGA2ox shares the highest identity (72.92%) with the GA2ox from monocot oil palm (Table S1) and the phylogenetic analysis showed that AcGA2ox falls into GA2ox protein class I and is clustered in the same sub-group with EgGA2ox (Fig. 1), while showing identities between 25.79% and 58.88% with the four GA2oxs from rice, also a monocot. On the other hand, AcGA2ox shares very high identity with all the five GA2oxs from the dicot tobacco, ranging from 58.12% to 60.06%. However, AcGA2ox shows the lowest identity with GA2ox protein class III AtGA2ox7 and AtGA2ox8, 23.59% and 26.35%, respectively (Table S1). Transient expression of AcGA2ox in protoplasts of Arabidopsis mesophyll indicates that it was localized in cytoplasm (Fig. S2).
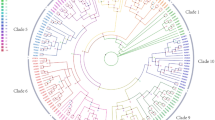
Maximum-likelihood phylogenetic tree based on comparison between pineapple GA2ox (AcGA2ox) protein sequence and GA2ox proteins from five other species, i.e., Arabidopsis (Arabidopsis thaliana), rice (Oryza sativa), tomato (Solanum lycopersicum), tobacco (Nicotiana tabacum) and oil palm (Elaeis guineensis).
The phylogenetic tree was created using MEGA6.06 (downloaded from the website http://www.megasoftware.net/mega.php). The three GA2ox classes (I, II, and III) are indicated. Protein names and the corresponding Genbank accession numbers of the proteins are: AcGA2ox(ACN30002), AtGA2ox1(CAB41007), AtGA2ox2(CAB41008), AtGA2ox3(CAB41009), AtGA2ox4(AAG51528), AtGA2ox6(AAG00891), AtGA2ox7(AAG00891), AtGA2ox8(ABE66080), OsGA2ox1(BAB40934), OsGA2ox2(BAC16751), OsGA2ox3 (BAC16752), OsGA2ox4 (AAU03107), OsGA2ox5(BAC10398), OsGA2ox6 (CAE03751), OsGA2ox7(BAG98459), OsGA2ox8(XP_006648857), SlGA2ox1(EF441351), SlGA2ox2(EF441352), SlGA2ox3(EF441353), SlGA2ox4(EF441354), SlGA2ox5(EF441355), NtGA2ox1(BAD17855), NtGA2ox2(BAD17856), NtGA2ox3(ABO70985), NtGA2ox4(AGL39429), NtGA2ox5(ABO70986), and EgGA2ox(AFS65097.1).
AcGA2ox expression is related to IB development in pineapple
Firstly, low temperature (LT, 5 °C) upregulates AcGA2ox and inhibits IB development in pineapple. LT enhances AcGA2ox transcript accumulation (Fig. 2A), and reduces GA4 levels by 26% on 1 day after treatment (DAT) and 37% on 9 DAT compared with the control (20 °C) (Fig. 2B), as measured by RT-PCR. Meanwhile, pineapples stored at LT for 14 d remain intact, while the control shows severe symptoms of IB (Fig. 2C,D). Secondly, exogenous abscisic acid (ABA) application enhances AcGA2ox transcription at ambient temperature (20 °C) (Fig. 3A), and decreases endogenous GA4 by 24% on 1 DAT and 14% on 9 DAT (Fig. 3B). At the same time, ABA treatment significantly inhibits IB incidence in pineapple fruits (Fig. 3C,D). However, exogenous GA4/7 treatment upregulates AcGA2ox, but the GAs biosynthesis inhibitor paclobutrazol downregulates it (Fig. S3).
Effects of low temperature on AcGA2ox gene expression, endogenous GA4 levels, and IB development.
For LT treatment, pineapple fruits were stored at 5 °C, with those stored at 20 °C serving as control. AcGA2ox gene expression in pulp of pineapple fruits was analyzed by semi-quantitative RT-PCR (A) while GA4 levels in the pulp were determined by ELISA (B). IB incidences (C) were evaluated and the pictures of symptoms (D) taken after 14 d of storage. The significance of observed differences between the control and LT treatment is indicated by a letter above the relevant bar (P ≤ 0.01). Standard errors are shown (B,C).
Effect of ABA on AcGA2ox gene expression, endogenous GA4 levels, and IB incidence in harvested pineapple fruits.
Fruits were sprayed with ABA solution at 200 mg.L−1, with distilled water as control. Following treatment, both ABA-treated and control fruit were stored at 20 °C. AcGA2ox gene expression in pulp of pineapple fruits was analyzed using semi-quantitative RT-PCR (A) while GA4 levels in the pulp were determined by ELISA (B). IB incidences (C) were evaluated and the pictures of symptoms (D) taken after 14 d of storage. Significant differences between the control and LT treatment are indicated by letters above each bar (P ≤ 0.01). Standard errors are shown (B,C).
AcGA2ox over-expressing Arabidopsis display phenotypes related to GAs deficiency
For ectopical expression in Arabidopsis, the 978 bp cDNA of AcGA2ox was cloned into the pCAMBIA1305.1 vector downstream of a CaMV 35S promoter (Fig. S4), which was then used to transform Arabidopsis plants, generating transgenic Arabidopsis lines stably overexpressing AcGA2ox (Fig. S5). The transgenic plants over-expressing AcGA2ox have significantly lower GA4 content (Fig. 4A) and seed germination rates (Fig. 4B,C) than wild-type Arabidopsis plants. However, when cultured on GA4/7-containing medium, the transgenic seeds show significantly higher germination rates (P ≤ 0.05), comparable to that of the wild type (Fig. 4B,C). In addition, the hypocotyl and root length of the transgenic line is significantly decreased compared to wild type, which is partly restored by exogenous GA4/7 (Fig.5A–C). In addition, AcGA2ox is constitutively expressed in transgenic plants, but not in wild type (Fig. S6), and AtGA3ox1 and AtGA5 are up-regulated in the transgenic plants (Fig. S6). These results confirm the function of AcGA2ox in regulating GAs levels.
GA4 contents in AcGA2ox over-expression Arabidopsis (A) and effects of exogenous GA4/7 on germination of seeds transgenic lines (B,C). GA4 levels in the seedlings were determined by ELISA 7 d after germination. For assay of seed germination, sterilized T3 transgenic seeds were sown on the MS culture medium, either complemented with GA4/7 (Sigma) at 25 mg.L−1 or without. Significance of differences is indicated by letters above the bars (P ≤ 0.05). WT: wild type of Arabidopsis. Over: transgenic Arabidopsis overexpresing AcGA2ox gene. Standard errors are shown (A,C).
Length of hypocotyls and roots of transgenic Arabidopsis seedlings over-expressing AcGA2ox in response to GA4/7.
GA4/7 at 25 mg.L−1 were applied by adding to the MS culture medium. Picture (A) was taken and length of hypocotyls and roots length (B,C) measured 15 d after germination. WT: wild type of Arabidopsis. Over: transgenic Arabidopsis overexpresing AcGA2ox gene. The scale bar on the upper right is 10 mm. Significant differences are indicated by letters above the bars (P ≤ 0.05). Standard errors are shown (B,C).
Polyphenol oxidases expression in response to factors affecting AcGA2ox gene expression and IB
As activities of polyphenol oxidases (PPO) were correlated with the degree of browning2, expression of polyphenol oxidase (PPO) in pineapple fruits was investigated. The results show that GA4/7 treatment enhances AcPPO expression, while low temperature and ABA decreases AcPPO expression (Fig. 6). This confirms that the AcGA2ox gene plays an important role in regulating IB in pineapple through the modulation of GAs levels.
Semi-quantitative PCR showing gene expression of polyphenol oxidase (PPO) in pulp of pineapple fruits in response to treatment with GA4/7, low temperature, and ABA.
For LT treatment, pineapple fruits were stored at 5 °C, with those stored at 20 °C serving as control. Fruit surfaces were treated with either GA4/7, ABA, or distilled water. Following treatment, all fruits were stored at 20 °C.
Discussion
GA2oxs are encoded by a multigene family15. Here we cloned a GA2ox from pineapple, an herbaceous and monocot plant. The protein sequence of AcGA2ox includes His-113, Asp-115, and His-272 in the 2OG-FeII_Oxy domain (Fig. S1). These residues are thought to associate with Fe2+ 23 at the catalytic site and are conserved in all known rice GA2ox proteins24. Although the sequence identity between AcGA2ox and 26 GA2oxs from five different plant species varies greatly, AcGA2ox shares the highest identity with (72.92%, Table S1), and clustered in the same sub-group as, EgGA2ox from oil palm (Fig. 1).
The GA2ox family is composed of two subfamilies differing in their substrate specificity, C19-GAs and C20-GAs17. C19-GA 2-oxidation is a major GA inactivation pathway in Arabidopsis19. C19 -GA2oxs hydroxylate the C-2 of active C19-GAs (GA1 and GA4) or C19-GA precursors (GA20 and GA9) to form biologically inactive GA8, GA34, GA51, and GA29, respectively24,25, while C20 -GA2oxs, a more recently discovered type of GA2oxs which includes AtGA2ox7 and AtGA2ox8, use C20-GAs as substrates17. Class I & II belong to the C19 -GA2oxs subfamily, while class III belongs to the C20 -GA2oxs subfamily21. Phylogenetic analysis shows that AcGA2ox falls into class I (Fig. 1), suggesting that it inactivates GAs by hydroxylating C19-GAs such as GA1 and GA4. AcGA2ox is localized in cytoplasm (Fig. S2) and both C20-GAs and C19-GAs are formed in cytoplasm26, which enables AcGA2ox to act quickly in response to the changes in internal and external environments.
Studies show that the expression of GA2ox in response to LT could differ strikingly. LT (4 °C) suppresses expression of AtGA2ox2 and increases the level of bioactive GAs in Arabidopsis seeds6, but LT upregulates expression of GA2ox genes in Arabidopsis seedlings, reducing GA levels and inhibiting the seedling growth7. Exposure to LT increases expression of PsGA2ox2 in pea plants27. Most importantly, under those cases, GA2oxs all function by negatively regulating the concentration of bioactive GAs. In this study, we show that LT (5 °C) upregulates expression of AcGA2ox and decreases levels of GA4, one of the major bioactive forms of GAs (Fig. 2), consistent with former studies on the mechanism of GA2oxs in regulating GAs.
Previous studies showed that GA3 application leads to development of IB in pineapple1,3. In this study, we further showed that ABA, the antagonist of GAs, effectively inhibits IB development in pineapple fruits stored in ambient temperature. Furthermore, exogenous ABA enhances AcGA2ox expression and reduces GA levels (Fig. 3). These results are significant for three reasons. Firstly, they confirm that bioactive GAs exceeding a certain level cause IB. Therefore, to keep physiologically healthy, a plant or an organ of plant should tightly maintain the concentration of bioactive GAs. Perhaps this is why exogenous GAs reduces the expression of GA20ox genes and stimulates the expression of GA2ox genes in rice11 and enhances the expression of GA2ox genes in Arabidopsis15,19. These reports are consistent with our results that GA4/7 quickly activates expression of AcGA2ox in pineapple fruits, which is just as quickly inactivated by paclobutrazol, an inhibitor of GAs biosynthesis28,29 (Fig. S3). This mechanism could also explain why overexpressing AcGA2ox from pineapple in Arabidopsis plants causes the Arabidopsis genes AtGA5 and AtGA3ox1 to be upregulated (Fig. S6): in order to tightly maintain GAs levels. Secondly, since GAs concentrations are regulated at both the level of hormone synthesis and through controlled inactivation, GA 2-oxidation is a major inactivating pathway for the plant hormone GAs19. These results also suggest that, in harvested pineapple, ABA antagonizes GAs partly by inactivating biosynthesis of active GAs. Thirdly, these results confirmed that, in harvested pineapple, AcGA2ox plays an important role in negatively regulating active GAs.
The roles of GA2oxs in different processes of plant development have been extensively elucidated. These processes include fruit growth21, underground growth19, seed germination, and vegetative and reproductive transitions20. Dwarfing is the most typical phenotype of plants overexpressing GA2oxs, such as AtGA2ox7 and AtGA2ox8 in Arabidopsis and tobacco17, poplar PtaGA2ox116 and PtGA2ox520 in poplar trees, spinach SoGA2ox3 in Nicotiana sylvestris30, plum PslGA2ox in Arabidopsis31, and grapevine VvGA2ox genes in Arabidopsis32. However, apart from morphological changes, there has been no report showing what happens to the plants or plant organs that no longer grow when GA2ox expression is upregulated. Here we showed that the enhanced AcGA2ox expression in harvested pineapple in response to low temperature and ABA is related to the inhibition of IB (Figs 2 and 3). Since gene transcript levels do not necessarily correspond to functional activity of enzymes, Arabidopsis overexpressing AcGA2ox was generated in order to show how the pineapple AcGA2ox gene regulates GAs levels (Figs S4 and S5). We showed that the transgenic plants overexpressing AcGA2ox display decreased GA4 levels, lower seed germination rates (Fig. 4), and shorter hypocotyls and roots (Fig. 5) than wild-type plants. All of these phenotypes are reversed by application of GA4/7. This strongly suggests that the phenotypes are a result of GAs deficiency. Our results are consistent with the phenotypes of Nicotiana sylvestris overexpressing GA2ox3 from spinach30 and the response of Arabidopsis overexpressing AcGA2ox to GAs is similar to that of rice overexpressing GA2ox625. These results confirm that AcGA2ox negatively regulates bioactive GAs concentration in harvested pineapple.
IB involves oxidation of phenolic compounds, and activities of polyphenol oxidases (PPO) were correlated with the degree of browning2,33. Promoters of two pineapple polyphenol oxidase (PPO) genes (PINPPO1 and PINPPO2), when transiently expressed in pineapple fruits and ectopically expressed in tobacco, were GA3 responsive1. Here we showed that the AcPPO gene was upregulated in response to GA4/7 (Fig. 6A) and downregulated in response to LT and ABA (Fig. 6B,C), suggesting that AcGA2ox regulates IB development by influencing active GAs levels in harvested pineapple. This is the first report showing the link between AcGA2ox and a physiological disorder.
In conclusion pineapple AcGA2ox reduces levels of bioactive GAs, leading to decreased transcription of AcPPO, which then results in decreased oxidation of phenolic compounds and contributes to inhibition of IB, the major physiological disorder of pineapple.
Methods
Plant materials and treatment
Pineapple (Ananas comosus [L.] Merr. cv ‘Comte de Paris’) fruits at commercial maturity were collected from a commercial plantation in Xuwen County, Guangdong Province and stored at low temperature (5 °C), with fruits stored at 20 °C as control. Sample pulp tissues were collected at 6 h, 12 h, 24 h and every 2 d or 3 d thereafter, frozen in liquid nitrogen and stored at −80 °C.
For ABA or GA4/7 treatment, solutions of ABA at 200 mg.L−1 or GA4/7 at 300 mg.L−1 was sprayed on the surface of pineapple until runoff. The control fruits were sprayed in the same way with distilled water. Following treatment, pineapples were stored at 20 °C. Samples of pulp tissues were collected at 6 h, 12 h, 24 h, 3 d, 6 d, and 9 d, frozen in liquid nitrogen and stored at −80 °C.
RNA preparation
Total RNA from pulp of pineapple was extracted following the method of Wan and Wilkins (1994)34 and RNA from Arabidopsis thaliana plants was extracted using Trizol reagent (Invitrogen, USA) according to the supplier’s instruction. Contaminant genomic DNA was digested by RNase-free DNaseІ.
Isolation of AcGA2ox full-length cDNA
First strand cDNA was synthesized using RevertAidTM First Strand cDNA Synthesis Kit according to the manufacturer’s protocol. cDNA was used as a template for amplifying a partial sequence of the AcGA2ox gene. The forward and reverse primers were 5′-GGA(G) TTC TTC(T) AAA(G) GTC (GT) A(G)TA(CGT) AAC(T) CA -3′ and 5′-TA(CT)A CA(G)C TCT T A(G)A AC(T)C TC(T)C CA(G)T T-3′, respectively. Conditions for PCR amplification: 35 cycles of 94 °C for 1 min, 45 °C for 1.5 min and 72 °C for 2 min.
The 5′- and 3′- ends of the AcGA2ox cDNA were obtained by RACE-PCR, using the 5′-Full RACE Kit and the 3′- RACE version2.0 (TaKaRa, Shiga, Japan). For the 5′-RACE, the primers for the 1st nested PCR and the 2nd nested PCR amplifications were: 5′-AGA GCT CCT TCT CGA CCT GTG GCA-3′ (2 × 2-5outer), 5′-ACC TCA ATG CTT CGT CCT CCA AC-3′ (2 × 2-5inner), respectively. Those for 3′-RACE were 5′-TCT GTT CCT CCT GAT CAA AGC TCT-3′ (2 × 2-3outer), 5′-TCT TCA TCA ATG TTG GCG ATT CA-3′ (2 × 2-3inner), respectively. The PCR products were separated in 1.0% (w/v) agarose gels, purified and ligated into the pMD-18T vector (TaKaRa, Shiga, Japan). The nucleotide sequences of the cDNA inserts were analyzed using the thermo sequenase dye terminator cycle sequencing kit and a 377 DNA sequencer (Perkin-Elmer Applied Biosystems).
Sequence analysis
DNA sequence data were analyzed using the programs provided by the National Centre of Biotechnology (NCBI) web site (http://www.ncbi.nlm.nih.gov). The BLASTN and BLASTP programs were used for the gene sequence homology search. Amino acid sequences of selected plant GA-2oxidase genes were aligned using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). A phylogenetic tree was constructed using maximum parsimony with 500 bootstrap replicates of MEGA6.06. ExPASy web site (http://cn.expasy.org/tools/) and programs therein were used for the prediction of amino acid features of AcGA2ox.
Gene expression analysis
Semi-quantitative RT-PCR was used for the analysis of gene transcript accumulation in pineapple fruits (AcGA2ox and AcPPO) and leaf tissues of Arabidopsis transgenic lines (AcGA2ox, AtGA5, AtGA3ox1) following the method of Matsushita et al.35 and Zhu et al.36. The primers (Table 1) were designed according to cDNA sequences. The actins from pineapple (AcActin) and Arabidopsis (AtActin) were PCR amplified as an internal control (Table 1).
Observation of IB severity
Three fruits from each treatment, one from each replicate, were cut every three days for monitoring the development of IB. Upon obvious onset of browning in flesh or core of pineapples from any treatment, all the remaining fruits (10–12 fruits per replicate) were then cut for observation of IB. IB incidence was calculated as percentage of fruits with symptoms of IB.
Determination of GA4 contents in pineapple tissue and Arabidopsis
GA4 from pineapple and Arabidopsis was extracted and purified according the method described by Talon and Zeevaart37. The total concentrations of GA4 were then determined following the method by Yang et al.38 using ELISA kits manufactured by Jiang Lai Bio-Technology, Shanghai, China)39.
Generation of overexpression construct of AcGA2ox
For ectopic expression of the AcGA2ox gene, the coding sequence was transferred to pCAMBIA1305.1 (kindly provided by prof. Yaoguang Liu from Key Laboratory of Plant Functional Genomics and Biotechnology, Education Department of Guangdong Province, South China Agricultural University, Wushan Road, Guangzhou 510642, China) at the Spe I and Pst I sites using T4 ligase (Invitrogen, USA), fused with 35S promoter. The construct pC-AcGA2ox was then transferred into Agrobacterium tumefaciens strains LBA4404 and transformed into Arabidopsis using the floral dip method40.
Screening of transgenic lines
Arabidopsis seeds from plants subjected to floral dip transformation were surface sterilized, sown onto 1% agar containing MS medium containing kanamysin (50 μg.ml−1) and selected for transgenic seedlings according the protocol described by Clough and Bent40. One week after germination, transformants had green and expanded cotyledons, and extended roots, while non-transformants showed yellow cotyledons and under-developed roots and died soon. The kanamycin-resistant transformants identified were then transplanted and grown in growth chambers under long days (16 h light/8 h darkness) and the day and night temperatures were 22 °C and 16 °C, respectively, for production of T1 seeds.
Transgene detection
DNA and mRNA extracted from the kanamycin-resistant transformants and T1, T2 and T3 plants were used as templates to perform PCR using AcGA2ox gene-specific primers AcGA2ox-F (5’-GACTAGTTCAAAATTGCCCAAGCCT-3’) and AcGA2ox-R (5’-AACTGCAGACCTCAACAACAACCATG -3’). All PCR products were visualised on a 1.1% agarose gel containing ethidium bromide (1 μg.ml−1).
Phenotype analysis of transgenic Arabidopsis plants over-expressing pineapple AcGA2ox gene
Seed germination was assayed following the method by Laval et al.41 with adaption. Sterilized T3 transgenic seeds were sown on MS culture medium, either complemented with GA4/7 (Sigma) at 25 mg.L−1or without. Seeds were incubated 2 d at 4 °C prior to the transfer to culture chamber at 22 °C with 16/8 h light/dark photoperiod. Germination was scored within 4 d of incubation. Length of hypocotyls was measured 15 d after germination.
Experiment design and statistical analysis
The experiments were completely randomized designs. Treatments were applied to three replications of 20 fruit for IB evaluation, three replications of 100 seeds for seed germination assay, and three replications of 20 seedlings for measurement of hypocotyls and root length. Data were analyzed by one-way analysis of variance (ANOVA). Mean separations were performed using the least significant difference method (LSD test). Statistically significant differences were assumed when their P values were ≤0.05 and very significant differences when P ≤ 0.01. Different letters above the bars in the figures indicate significance at the 0.05 level (with lower case letters) or 0.01 level (with upper case letters).
Additional Information
How to cite this article: Zhang, Q. et al. Mechanism of internal browning of pineapple: The role of gibberellins catabolism gene (AcGA2ox) and GAs. Sci. Rep. 6, 33344; doi: 10.1038/srep33344 (2016).
References
Zhou, Y. C. et al. Transcriptional regulation of a pineapple polyphenol oxidase gene and its relationship to blackheart. Plant Biotechnol. J. 1, 463–478 (2003).
Pusittigul, I., Kondo, S. & Siriphanich, J. Internal browning of pineapple (Ananas comosus L.) fruit and endogenous concentrations of abscisic acid and gibberellins during low temperature storage. Sci. Hortic. 146, 45–51 (2012).
Yu-Chan, Z. & Xing-Jie, T. Mechanism of blackheart development induced by low temperature and gibberellic acid in pineapple fruit. Acta Hortic. 425, 587–594 (1997).
Gong, D. et al. Effects of low temperature storage on blackheart incidence and quality. Maintenance of pineapple fruits. Transactions of the CSAE 26, 365–369. (in Chinese with English abstract) (2010).
Hong, K. et al. Quality changes and internal browning developments of summer pineapple fruit during storage at different temperatures. Sci. Hortic. 151, 68–74(2013).
Yamauchi, Y. et al. Regulation of gibberellin biosynthesis by low temperature in imbibed Arabidopsis seeds. Plant Cell 16, 367–378(2004).
Achard, P. et al. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20, 2117–2129 (2008).
Hedden, P. & Kamiya, Y. Gibberellin biosynthesis, enzymes, genes and their regulation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 431–460(1997).
Lange, T. Molecular biology of gibberellin synthesis. Planta 204, 409–419 (1998).
Phillips, A. L. et al. Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol. 108, 1049–1057 (1995).
Sakamoto, T. et al. Expression of a gibberellin 2-oxidase gene around the shoot apex is related to phase transition in rice. Plant Physiol. 125, 1508–1516 (2001).
Ross, J. J. et al. Genetic regulation of gibberellin deactivation in Pisum. Plant J. 7, 513–523 (1995).
Lester, D. R., Ross, J. J., Smith, J. J., Elliott, R. C. & Reid, J. B. Gibberellin 2-oxidation and the SLN gene of Pisum sativum. Plant J. 19, 65–73 (1999).
Martin, D. N., Proebsting, W. M. & Hedden, P. The SLENDER gene of pea encodes a gibberellin 2-oxidase. Plant Physiol. 121, 775–781 (1999).
Thomas, S. G., Phillips, A. L. & Hedden, P. Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc. Natl. Acad. Sci. USA 96, 4698–4703(1999).
Busov, V. B. et al. Activation tagging of a dominant gibberellin catabolism gene (GA 2-oxidase) from poplar that regulates tree stature. Plant Physiol. 132, 1283–1291 (2003).
Schomburg, F. M., Bizzell, C. M., Lee, D. J., Zeevaart, J. A. D. & Amasino, R. M. Overexpression of a novel class of gibberellin 2-oxidases decreases gibberellin levels and creates dwarf plants. Plant Cell 15, 151–163 (2003).
Appleford, N. E. J. et al. Decreased shoot stature and grain {alpha}-amylase activity following ectopic expression of a gibberellin 2-oxidase gene in transgenic wheat. J. Exp. Bot. 58, 3213–3226 (2007).
Rieu, I. et al. Genetic analysis reveals that C19-GA 2-oxidationisa major gibberellins in activation pathway in Arabidopsis. Plant Cell 20, 2420–2436(2008).
Gou, J. et al. Tissue-specific expression of Populus C(19) GA 2-oxidases differentially regulate above- and below-ground biomass growth through control of bioactive GA concentrations. New Phytologist 192, 626–639(2011).
Serrani, J. C., Sanjuán, R., Ruiz-Rivero, O., Fos, M. & García-Martínez, J. L. Gibberellin regulation of fruit set and growth in tomato. Plant Physiol. 145, 246–257 (2007).
Wang, Y., Zhang, L. & Zhu, S. 1-methylcyclopropene (1-MCP)-induced protein expression associated with changes in Tsai Tai (Brassica chinensis) leaves during low temperature storage. Posthar. Biol. Technol. 87, 120–125(2014).
Valegård, K. et al. Structure of a cephalosporin synthase. Nature 394, 805–809(1998).
Sakamoto, T. et al. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 134, 1642–1653 (2004).
Lo, S. F. et al. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell 20, 2603–2618 (2008).
Olszewski, N., Sun, T. P. & Gubler, F. Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell 14 Suppl, S61–80 (2002).
Stavang, J. A., Junttila, O., Moe, R. & Olsen, J. E. Differential temperature regulation of GA metabolism in light and darkness. J. Exp. Bot. 58, 3061–3069(2007).
Lee, E. H., Byun, J. K. & Wilding, S. J. A new gibberellin biosynthesis inhibitor, paclobutrazol (PP333), confers increased SO2 tolerance on snap bean plants. Environ. Exp. Bot. 25, 265–275(1985).
Shiow, Y., Wang, S. Y., Sun, T. & Faust, M. Translocation of paclobutrazol, a gibberellin biosynthesis inhibitor, in apple seedlings. Plant Physiol. 82, 11–14(1986).
Lee, D. J. & Zeevaart, J. A. Molecular cloning of GA 2-oxidase3 from spinach and its ectopic expression in Nicotiana sylvestris. Plant Physiol. 138, 243–254(2005).
El-Sharkawy, I. et al. Identification and genetic characterization of a gibberellin 2-oxidase gene that controls tree stature and reproductive growth in plum. J. Exp. Bot. 63, 1225–1239 (2012).
Giacomelli, L. et al. Gibberellin metabolism in Vitis vinifera L. during bloom and fruit-set: functional characterization and evolution of grapevine gibberellin oxidases. J. Exp. Bot. 64, 4403–4419 (2013).
Raimbault, A.-K. et al. Polyphenol oxidase and peroxidase expression in four pineapple varieties (Ananas comosus L.) after a chilling injury. J. Agric. Food Chem. 59, 342–348(2011).
Wan, C. Y. & Wilkins, T. A. A modified hot borate method significantly enhances the yield of high quality RNA from cotton (Gossypium hirsutum L.). Anal. Biochem. 223, 7–12 (1994).
Matsushita, A., Furumoto, T., Ishida, S. & Takahashi, Y. AGF1, an AT-hook protein, is necessary for the negative feedback of AtGA3ox1 encoding GA 3-oxidase. Plant Physiol. 143, 1152–1162 (2007).
Zhu, X., Wang, A., Zhu, S. & Zhang, L. Expression of ACO1, ERS1 and ERF1 genes in harvested bananas in relation to heat-induced defense against Colletotrichum musae. J. Plant Physiol. 168, 1634–1640(2011).
Talon, M. & Zeevaart, J. A. D. Gibberellins and stem growth as related to photoperiod in Silene armeria L. Plant Physiol. 92, 1094–1100 (1990).
Yang, J., Zhang, J., Wang, Z., Zhu, Q. & Wang, W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol. 127, 315–323 (2001).
Zhang, Y. L. et al. Peripheral blood MDSCs, IL-10 and IL-12 in children with asthma and their importance in asthma development. PLoS ONE 8, e63775, doi: 10.1371/journal.pone.0063775 (2013).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Laval, V. et al. Seed germination is blocked in Arabidopsis putative vacuolar sorting receptor (atbp80) antisense transformants. J. Exp. Bot. 54, 213–221 (2003).
Acknowledgements
This work was supported in part by Special Fund for Agro-scientific Research in the Public Interest from China Ministry of Agriculture (201203021) and Guangdong Province Science and Technology Plan Project (2016A020210077). Professor Yaoguang Liu and Chuxiong Zhuang from Key Laboratory of Plant Functional Genomics and Biotechnology, Education Department of Guangdong Province, South China Agricultural University is thanked for providing part of the experimental facilities. Dr. Jeffrey Zuber from University of Rochester is thanked for critical reading of and grammatical improvement on the manuscript.
Author information
Authors and Affiliations
Contributions
S.Z. designed and oversaw the work. Q.Z. and X.R. performed the transgenic experiment. L.Z. participated in sequence analysis. Q.Z. and C.H. performed the pineapple experiment. F.Y. isolated the full-length sequence of AcGAox. S.Z. made the figures and tables, and wrote the manuscript. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, Q., Rao, X., Zhang, L. et al. Mechanism of internal browning of pineapple: The role of gibberellins catabolism gene (AcGA2ox) and GAs. Sci Rep 6, 33344 (2016). https://doi.org/10.1038/srep33344
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33344
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.