Abstract
Increased p66Shc expression has been associated with diabetic nephropathy (DN). However, whether p66Shc can serve as a potential biomarker for tubular oxidative injury in DN is unknown. We measured the expression of p66Shc in peripheral blood monocytes (PBMs) and renal biopsy tissues from DN patients and then analysed the relationship between p66Shc expression and the clinical characteristics of patients with DN. Patients were divided into 4 groups (class IIa, class IIb, class III and the control group). qPCR, Western blotting and immunohistochemistry were performed. The results showed that both p66Shc and p-p66Shc expression significantly increased in PBMs and kidney tissues of DN patients. Moreover, Spearman’s correlation and multiple regression analyses were carried out. A positive relationship between the p66Shc expression and oxidative stress was found. p66Shc and oxidative stress were significant predictors of the degree of tubular damage. In addition, p66Shc expression was positively correlated with the concentrations of β-NAG, UACR and 8-OHdG, low-density lipoprotein and blood glucose levels, and duration of diabetes in patients with DN from class IIa to class III. These data indicated that increased expression of p66Shc may serve as a therapeutic target and a novel biomarker of DN.
Similar content being viewed by others
Introduction
Diabetic nephropathy (DN) is a severe microangiopathic complication in patients with both type 1 and type 2 diabetes mellitus. A number of risk factors have been associated with the progression of DN, including glomerular hypertension, proteinuria, hyperlipidaemia and genetic predisposition1. Studies carried out over the last 3 decades have indicated that there are a series of underlying mechanisms in the progression of kidney injury in DN. In recent years, excessive generation of reactive oxygen species (ROS) has emerged as the major pathogenetic denominator in the progression of DN2. During hyperglycaemia, excessive amounts of ROS are produced from both the NAPDH system and mitochondrial sources, leading to the formation of vascular lesions via metabolic modifications of target tissue molecules and disturbances in the intrarenal haemodynamics. As ROS induce renal injury, it is anticipated that renal tissue injury will be reflected in compromised renal functions3,4. Because ROS are the major inducers of renal injury in microvascular complications of diabetes, the molecules or the pathways involved in their generation could serve as therapeutic targets to ameliorate the progression of DN or alternatively could serve as biomarker(s) to monitor the progression of DN. Therefore, we explored the relevance of p66Shc in DN and determined whether it could serve as a biomarker during the progression of the renovascular complications of diabetes.
p66Shc is a member of the adaptor protein family, which is encoded by four loci in mammals. Three isoforms are encoded by ShcA, which include proteins with relative molecular weights of 46, 52 and 66 kDa. Among them, p46/p52 are ubiquitously distributed and are expressed in various tissues, while p66Shc has restricted tissue-specific expression5,6. All these proteins contain a phosphotyrosine binding domain (PTB), a collagen homology domain-1 (CH1) and a Src homology 2 domain (SH2)6. The p66Shc protein is distinct because it has an additional N-terminal region named CH2, which is responsible for its redox properties and is involved in lifespan regulation and apoptosis7. The structural features of Shc isoforms suggest that they play a role in diverse cellular functions; for example, p46Shc and p52Shc are involved in the transmission of mitogenic signals from tyrosine kinases to RAS proteins, while p66Shc is primarily associated with mitochondrial ROS production, oxidative stress and induction of apoptosis5. In addition, treatment of 293A cells (a human embryonic kidney cell line) with high glucose (HG) increased p66Shc expression, while there was no change in p46/p52 expression8. This suggests that p66Shc is specifically relevant to the pathogenesis of DN. In addition, several different studies have indicated that p66Shc is involved in various chronic diseases that are secondarily due to oxidative damage9,10,11,12. Furthermore, there are many in vivo and in vitro studies implicating p66Shc in the progression of DN via the modulation of mitochondrial ROS production, leading to oxidative stress in the kidney13,14. Our previous studies also suggest that expression of both p66Shc and the phosphorylated form of p66Shc (p-p66Shc) is increased in diabetic mouse models and associated with oxidative injury of the tubular cells of the kidney in DN15. Interestingly, genetic loss of the p66Shc gene in mice partially prevented glucose intolerance and premature death16. Moreover, Menini et al. demonstrated that mice with a p66Shc deletion showed less severe pathological renal lesions and lower proteinuria levels, glomerular sclerosis index and matrix accumulation under hyperglycaemic conditions17. Similarly, Pagnin et al. noted that diabetes induced p66Shc gene expression in circulating peripheral blood monocytes (PBMs) and demonstrated that the up-regulation of p66Shc was associated with increased oxidative stress18. Except for the studies on PBMs, very limited information is available on the status of p66Shc expression in patients with DN, and few reports have established a relationship between p66Shc expression and the clinical characteristics of patients with DN.
In view of the above considerations, we investigated the p66Shc expression in kidney tissues in patients with DN and established a correlation between p66Shc expression and the clinical characteristics of these patients. We noted that the p66Shc and p-p66Shc expression in PBMs as well as renal tissues was significantly increased in patients with DN compared to controls, and the up-regulated p66Shc and p-p66Shc expression was associated with increased ROS production, which was linked to cortical tubular lesions, suggesting that p66shc may serve as a new biomarker of tubular injury in patients with DN.
Results
P66Shc expression in patients with DN
The clinical characteristics of the DN patients and controls in this study are shown in Table 1. There were no significant differences between DN patients and controls in terms of age, sex, body mass index (BMI), alanine aminotransferase (ALT), aspartate aminotransferase (AST), high-density lipoprotein cholesterol (HDL-C), creatinine (Cr), systolic blood pressure (SBP), and diastolic blood pressure (DBP). Compared with the controls, the DN patients showed significantly increased levels of blood triglycerides, the estimated glomerular filtration rate (eGFR), low-density lipoprotein cholesterol (LDL-C), blood glucose, haemoglobin A1c (HbA1C), uric acid and 24 h urine protein excretion. In sharp contrast, serum albumin was dramatically decreased in DN patients. Interestingly, even in different DN groups, these physicochemical parameters are different. For example, the level of serum albumin was significantly decreased in class III versus class IIa and IIb. There were no significant differences in the other blood or urine parameters and in the clinical characteristics between the four groups.
As shown in Table 2 and Fig. 1B, the p66Shc protein level in PBMs of the DN groups was dramatically increased compared with the control group (p < 0.01). Between different DN groups, p66Shc mRNA levels in class IIb and class III were significantly higher than those of class IIa (p < 0.01). In addition, p66Shc mRNA levels in class III were higher than those of class IIb (Fig. 1A) (p < 0.01). The p66Shc and p-p66Shc protein levels were also increased in DN patients (Fig. 1B–E), while no significant differences in the ratio of p66Shc to p-p66Shc protein expression in each group was observed (Fig. 1F). To further analyse the p66Shc protein expression in PBMs of DN patients, a correlation analysis was carried out to assess whether there was an association with the development of DN. The results showed that the p66Shc protein expression was positively correlated with the duration of diabetes (Fig. 1G), levels of triglyceride (Fig. 1H), LDL-C (Fig. 1I), HbA1C (Fig. 1J) and blood glucose (Fig. 1K). No correlation was observed with the SBP (Fig. 1L). Similar results were also observed for p-p66Shc expression (Supplement Fig. 1A–F). The coefficient of correlation (R) of the above parameters is included in Table 2.
p66Shc expression in the PBMs of DN patients was determined by real-time PCR analyses (A). Western blot analyses of p66Shc and p-p66Shc protein expression in PBMs (B,C). (D,E) Quantification of average band intensity of Western blots (Control: n = 36; Class IIa: n = 15; Class IIb: n = 18; Class III: n = 17). (F) Bar graph represents the ratio of p-p66Shc to p66Shc expression, as assessed by quantification of relative band intensity. Control: n = 36; Class IIa:n = 15; Class IIb:n = 18;Class III:n = 17. *p < 0.01 versus control; #p < 0.05 versus class IIa; @p < 0.01 versus IIb. Values are shown as the mean ± SD. Correlation analysis of the expression level of p66Shc in PBMs of patients with DN and duration of diabetes (G), triglycerides (H), LDL-C (I), HbA1C (J), blood glucose (K) and SBP (L). Values are means ± E.
Pathological lesions and the correlation between tubular interstitial damage and oxidative stress
The morphological changes in the renal tissues of DN patients, including the glomerular and tubulointerstitial compartments, were assessed by HE, PAS and PASM staining. Light microscopy showed that samples from DN class II had mild mesangial expansion, while the mesangial expansion was more than 25% of the total mesangium in class III samples. Furthermore, Kimmelstiel-Wilson lesions were observed in class IIb and III tissues (Fig. 2A, upper panels). In addition, dihydroethidium (DHE) staining was performed in the sections from the renal biopsy of DN patients to measure oxidative stress, which is reflective of the ROS levels. Compared to the control group, significantly higher ROS levels were observed in the kidneys of patients with DN, which was accompanied by a higher degree of renal pathological changes (Fig. 2A, lower panels), and the increase in ROS levels positively correlated with renal pathological changes.
(A) The histological changes in renal biopsies of DN patients were assessed by HE PAS and PASM staining. Scale bar with black color 50 μM (magnification ×200). DHE staining indicates the oxidative stress in kidney tissues Scale bar with white color 10 μM (magnification ×40). (B) The bar graphs represent tubular interstitial damage scores of the renal biopsies from DN patients and controls. (C) Fluorescence intensity of DHE staining reflects kidney oxidative stress. *p<0.01 versus control; #p < 0.05 versus IIa; @p < 0.01 versus IIb. (D) The analysis of the correlation between tubular interstitial damage score and kidney oxidative stress (r = 0.732, p < 0.01).
We then determined the tubulointerstitial damage score in the renal tubular compartment as previously described19. The total score for a 6 point scoring criteria in DN patients was determined. The control group had a score of 0-1 points, while scores of 1 to 4 points and 3 to 6 points were observed for class II and class III DN patients, respectively. Statistical analysis showed that there were significant differences between the DN groups and the control group and class IIa versus class III groups (p < 0.01), but no significant differences were found between class IIa versus IIb and class IIb versus III (Fig. 2B). Semiquantitative analysis demonstrated that oxidative stress intensity (ROS levels) in the kidneys of DN class III patients was significantly higher than that in class IIa and IIb, and there was no significant difference between class IIa and class IIb (Fig. 2C). Furthermore, the tubular interstitial damage score was positively correlated with the renal oxidative stress in DN patients (r = 0.653, p < 0.01) (Fig. 2D).
p66Shc expression increased in the kidneys of DN patients and was correlated with oxidative stress
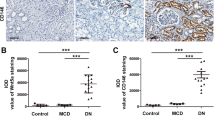
p66Shc protein expression in the kidneys of patients with DN was assessed by immunohistochemical (IHC) staining with mouse monoclonal anti-p66Shc and anti-phospho-p66Shc (Ser36) antibodies. IHC staining revealed a dramatic increase in p66Shc and p-p66Shc expression in DN patients compared with the control group (p < 0.01). The increase was predominantly confined to the renal proximal tubules, and very little expression was observed in the glomerular mesangium (Fig. 3A). Further analysis revealed that the expression of p66Shc and p-p66Shc was accompanied by an increase in severity of renal lesions in DN patients, and expression up-regulated in the class IIb and class III groups (Fig. 3B,C). In addition, correlation analyses showed a positive relationship between p-p66Shc expression and the relative ROS levels (r = 0.774) (Fig. 3D). Multiple regression analysis found a significant relative correlation among p66Shc, oxidative stress and tubular oxidative damage (Fig. 3E). The regression coefficient p-p66Shc (β1) contributed to tubular oxidative injury is 5.100, P = 0.007.The regression coefficient ROS (β2) contributed to tubular oxidative injury is 3.364, P = 0.041 (Fig. 3E). Furthermore, for analyzing the levels of p66Shc and p-p66Shc expression in different groups of pathological classification (IIa to III) in DN patients by the ANOVA analysis. A significant difference compared to control was found. Further investigation showed a significant difference was also seen on the degree of oxidative damage or tubular interstitial damage in the different pathological classification groups (Fig. 3G–I).
(A) Immunostaining of p66Shc and p-p66Shc in renal biopsy tissues (magnification ×200). (B,C) IHC was performed. Relative immunostaining density of p66Shc and p-p66Shc in the kidney tissues of DN patients in each of the groups. *p < 0.01 versus control; #p < 0.01 versus IIa; @p < 0.01 versus class IIb. Values are shown as the mean ± SD. (D) Spearman’s correlation analyses showed the association between p66Shc expression by IHC and oxidative stress, as assessed by DHE staining. (E) Result of multiple regression model of showed the contribution of p66shc, oxidative stress to tubular injury score. (F–I) The levels of p-p66Shc by IHC and p66Shc expression by Western blot analysis, the degree of oxidative injury and tubular interstitial damage in kidney of DN patients were different compared to those of controls on different groups of pathological classification (IIa to III) in patients with DN.
p66Shc expression was positively correlated with tubular damage and various clinical characteristics in DN patients
To test the relationship between p66Shc expression and tubular injury in DN patients, we first assessed the tubular damage in each group by measuring the urine levels of N-acetyl-β-glucosaminidase (β-NAG), urine albumin-to-creatinine ratio (UACR) and 8-OHdG in each group. The results showed that in the DN groups, the concentration of β-NAG, UACR and 8-OHdG increased with severity of the disease from DN class IIa to class III (Fig. 4A–C). Further analyses demonstrated that p66Shc expression in the kidneys of DN patients was positively correlated with tubulointerstitial damage (Fig. 4D), renal oxidative damage degree (ROS level) as detected by DHE staining (Fig. 4E), and urinary levels of β-NAG (Fig. 4F) and UACR (Fig. 4G). In addition, the linear regression analysis demonstrated that p66Shc and p-p66Shc expression in the kidneys of DN patients was positively correlated with the level of urinary 8-OHdG (Fig. 4H). Furthermore, analysis also revealed that p66Shc expression in the kidney and PBMs in DN patients were positively correlated (Fig. 4I). In the other hand, similar results were observed in the correlation of p-p66Shc expression in the kidney and the clinical indicators in DN patients (Supplement Fig. 2A–F).
(A) Bar graph represents the levels of urine β-NAG in patients with DN in each group. (B) The concentration of urine albumin: creatinine ratio (UACR) in patients with DN in each group. (C) Levels of urine 8-OHdG in each group. (D) Correlation analysis indicated that p66Shc expression in renal tissues of DN patients and the tubular interstitial damage score were positively correlated (r = 0.762, p < 0.01). (E) The p66Shc expression levels and the kidney oxidative stress were also positively correlated (r = 0.812, p < 0.01). (F) A positive correlation was found between the expression of p66Shc and urine β-NAG level (r = 0.812, p < 0.01). Correlations were also found for the concentration of UACR (G) (r = 0.830, p < 0.01) and urine 8-OHdG levels (H) (r = 0.874, p < 0.01) and p66Shc expression. The analysis of the correlation between the expression of p66Shc in the kidneys and PBMs is shown (I) (r = 0.632, p < 0.01). Values are shown as the mean ± SD. r: correlation coefficient.
Discussion
Excessive intracellular ROS generation in diabetes is considered a common pathway in hyperglycaemia-induced renal injury. The oxidative stress in diabetes patients is relatively high compared to healthy individuals, and it increases proportionately with the severity of the disease20. p66Shc, an oxidative stress response protein, is known to modulate mitochondrial ROS production. However, the relationship between p66Shc expression and the clinical characteristics of patients with DN had not been explored. This study reports three major findings. First, p66Shc expression was found to be increased in both PBMs and kidney tissues of DN patients. Second, there was a good correlation between the increased expression of p66Shc protein in the kidney tissues and in the PBMs of DN patients with renal oxidative stress. Third, we noted that p66Shc expression was positively correlated with tubular injury in patients with DN. These findings suggested that the increased expression of p66Shc may contribute to the progression of DN in patients with diabetes, and p66Shc in PBMs may serve as a potential biomarker to monitor the progression of DN in the future.
Recent studies have indicated that increased p66Shc expression is associated with ROS generation in human aortic endothelial cells exposed to high glucose (HG) conditions8. Other reports found that inhibition of p66Shc can reduce superoxide generation, maintain endothelial homoeostasis and alleviate kidney damage in diabetes and HG conditions13. However, it is unclear whether measuring the expression of oxidative stress-related adaptor proteins, such as p66Shc in the PBMs of DN patients, can serve as a marker of kidney damage. Urine 8-OHdG concentration may serve as an oxidative stress biomarker in various kidney diseases, but it lacks sensitivity and specificity for the early stages of DN. In this regard, DHE staining may serve as a marker for oxidative stress damage in renal biopsy tissues of DN patients, but it may not be practical because collecting kidney specimens during the early stages of DN could be a major impediment. Interestingly, expression of p66Shc in the PBMs and kidneys of patients with DN both had a positive correlation with the protein expression of p66Shc and p-p66Shc, tubular damage and oxidative stress. Likewise, enhanced p66Shc mRNA expression in the PBMs was associated with oxidative stress, indicating that measurement of p66Shc mRNA or p66Shc protein expression in PBMs may be a simple and a feasible potential biomarker for kidney oxidative stress and may be an important index gauging the progression of DN in diabetic patients.
In the present study, we also noted that the expression of p66Shc in PBMs and renal tissues of DN patients was positively correlated with the duration of diabetes, levels of triglycerides, HbA1C, LDL-C and blood glucose and tubular interstitial damage and renal oxidative stress (Figs 1, 2, 3 and Table 2). It is known that diabetes of long duration, poor glycaemic control, hypertension, and hyperlipidaemia are significant risk factors for the progression of DN21. Undoubtedly, HbA1C level is an indicator of chronic hyperglycaemia in diabetic kidney disease, and the clinical presentation has a strong relationship with oxidative stress in diabetic conditions22,23. Similarly, it is known that LDL-C levels are modified by oxidative stress in hyperglycaemic conditions24,25. Kim et al. found increased expression of p66Shc by LDL via alteration of CpG hypomethylation26. In addition, oxidized LDL (oxLDL) promoted p66Shc phosphorylation, while knockout of p66Shc decreased oxLDL-induced ROS generation and prevented the oxidative damage in endothelial cells27. These data indicate that the p66Shc expression in the PBMs may be a predictive marker of the progression of renal oxidative damage in DN patients.
Analysis of the relationship between tubular interstitial damage and renal oxidative stress indicated that the tubular interstitial injury score was increased in different categories of DN (Fig. 2). Previously, in vivo and in vitro studies in our laboratory demonstrated that the p66Shc expression was confined to the proximal tubular cells, and its expression was up-regulated in HK-2 cells (a human proximal tubular cell line) when exposed to high-glucose conditions and angiotensin II15. In the present study, we also found that the p66Shc and p-p66Shc were predominantly expressed in the renal tubular cells, and little expression was observed in the glomerular mesangium in renal biopsy tissues from DN patients. The expression of p66Shc and p-p66Shc was significantly increased in kidneys of DN class IIa, IIb and III. In addition, the increased p66Shc expression in kidneys was positively correlated with the degree of tubular damage and the levels of urinary β-NAG, 8-OHdG and UACR (Figs 3, 4), suggesting that renal tubular oxidative injury is probably modulated by p66Shc during the progression of DN. On the other hand, we further proved that there were a notable correlation between p66Shc expression and relative ROS level in kidney tissues of DN patients (Fig. 3D) or p-p66Shc, oxidative injury (ROS levels) and tubular interstitial damage, respectively (Fig. 3E). In addition, by ANOVA analysis, compared to control, a significant difference were observed on p-p66Shc expression in pathological classification IIa, II b and III of DN patients, respectively, Also similarly results was found in that of p66Shc expression, oxidative damage and tubular interstitial damage (Fig. 3F–I). This date indicated that the tubular oxidative damage in kidney of DN patients was associated with increased p66Shc or p-p66Shc expression.
Renal tubular damage plays a key role in the pathogenesis of DN, which may be independent of glomerular lesions28,29. Moreover, early tubular proliferation induced by oxidative stress in hyperglycaemic environments may contribute to the glomerular hyperfiltration30; thus, we also examined the relationship between p-p66Shc expression and renal functions. Unfortunately, there was no positive correlation (Table 2), which suggested that we may need to increase the sample size in future studies. Certainly, the p66Shc expression in PBMs was positively correlated with renal injury (Fig. 4). Therefore, it is possible that the PBMs with increased p-p66Shc levels invaded the kidneys, resulting in renal oxidative injury by locally generated ROS in the kidney tissues.
The mechanisms that mediate up-regulation of p66Shc expression and its phosphorylation in DN are not fully understood. However, the epigenetic regulation of p66Shc transcription is well known31. Cross-talk between p53 and p66Shc was observed in experimental models of diabetes32, in which p53 binds to the putative p53 binding sequence in the p66Shc promoter and then induces p66Shc transcription33. In addition, Sirt1 also binds to the p66Shc promoter, which could directly inhibit p66Shc transcriptional activity through epigenetic chromatin modification8. Furthermore, Sirt1 also inhibits p53 transcriptional activity induced by HG, which may affect the p66Shc expression34. In addition, activated protein C exerts its antioxidant effects by reversing glucose-induced hypomethylation and hyperacetylation of the p66Shc promoter5, suggesting that both p53 and Sirt1 are key regulators of p66Shc gene transcription. However, activated p53 and decreased Sirt1 expression have been found in DN in vivo and in vitro. Increased expression of p53 was found in the kidneys of STZ-treated rats and db/db diabetic mice35. OxLDL may trigger the phosphorylation and activation of p53, as demonstrated in HK-2 cells36. Additionally, it is known that HG directly attenuates the deacetylase activity of Sirt1 in HK-2 cells37. Thus, activation of p66Shc transcription in PBMs and the kidney tissues of DN patients may be a result of up-regulation of p53 and decreased activity of Sirt1. The next question that needs to be addressed is the mechanism underlying the increased phosphorylation of p66Shc in DN patients. It is well known that PKC-β induces phosphorylation of p66Shc38, and HG increases the synthesis of ANG II39. Our previous studies indicated that HG and Ang II could activate PKC-βand PKC-δ, which then phosphorylate p66Shc at the Ser36 residue. The phosphorylated p66Shc thus becomes a target for the prolyl isomerase Pin1, which recognizes the proline residue following the phosphorylated serine residue. After phosphorylation, p66Shc translocates into the mitochondria, and ROS generation is increased, leading to tubular oxidative injury15,40. In addition, ERK has been shown to phosphorylate p66Shc at Ser36 in tubular cells41, and ET-1 can also induce MEK/ERK-dependent p66Shc serine phosphorylation42, indicating that many of these above events might participate in phosphorylation of p66Shc, resulting in tubular oxidative injury in patients with DN.
Taken together, our study shows that increased expression of p66Shc may play a prominent role in renal tubular oxidative injury in the progression of DN. Moreover, p66Shc expression in PBMs may serve as a potential biomarker to monitor DN progression. Our study also indicates that inhibition of p66Shc may be a novel therapeutic strategy in the amelioration of renal tubular injury in DN.
Materials and Methods
Participants
Fifty patients with DN and thirty-six non-DN controls (minimal change disease) were recruited with written consent. All procedures were carried out in accordance with the approved guidelines. The DN patients were categorized into class IIa, IIb and III according to the 2010 pathologic classification of DN43. Class I and class IV were excluded because they have mild or nonspecific light microscopy changes or present with advanced diabetic glomerulosclerosis. The class IIa group included 15 patients (8 males and 7 females), aged 32–48 years (mean: 46.25 ± 8.53 years); the class IIb group included 18 patients (9 males and 9 females), aged 33–56 years (mean: 44.29 ± 9.250 years); and the class III group included 17 patients (8 males and 9 females), 42–58 years (mean: 51.14 ± 5.047 years). The control group included 36 patients (18 male and 18 females), aged 32–58 years (mean: 42.2 ± 8.066 years). Patients were diagnosed in the Second Xiangya Hospital, Central South University, according to the World Health Organization diagnostic criteria for type 2 diabetes. Patients did not use adrenal cortical hormones or immunosuppression drugs. The institutional review board and the administrators of the Department of Nephrology in Second Xiangya Hospital approved the protocol for this study. Informed consent was obtained from all the participants.
Biochemical analysis of blood and urine
The liver and renal functions, serum triglycerides, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) as well as blood glucose levels were analysed using standard automated enzymatic methods (Hitachi 912 automated analyser). N-acetyl-β-D-glucosaminidase (β-NAG) was determined by an automated colorimetric method (Pacific Biomarkers, Inc.). The concentration of 8-hydroxy-2′-deoxyguanosine (8-OHdG), a critical biomarker of oxidative stress and DNA damage in urine, was measured by ELISA (8-OHdG Check, Nikken Foods, Fukuroi, Shizuoka, Japan) by following the instructions provided by the vendor44.
Peripheral blood monocyte preparation
Peripheral blood (6 ml) was collected from various patients and then mixed with 6 ml of Dulbecco’s phosphate-buffered saline (PBS). The samples were centrifuged at 2,000 rpm for 30 min for separation into different layers. The turbid white layer containing the mononuclear blood cells was collected, rinsed with PBS twice and centrifuged at 1,500 rpm for 10 min. The mononuclear cells were then incubated in RPMI 1640 culture medium (Gibco BRL, Grand Island, USA) with 10% foetal calf serum (Invitrogen, Carlsbad, CA) at 37 °C in a 5% CO2 atmosphere. The adherent cells were collected and subjected to flow cytometry following staining with a CD-14 monoclonal antibody (Santa Cruz Biotechnology Inc., Texas, USA).
Quantitative real-time polymerase chain reaction
Total RNAs of the PBMs were prepared using TRIzol (Invitrogen). First-strand cDNAs were prepared by two-step RT-PCR (Fermentas Life Science). Relative gene expression was determined using SYBR Green quantitative real-time PCR assays on a 7500 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA). The cycling conditions were as follows: denaturing at 50 °C for 2 min, 95 °C for 10 min, then 40 cycles of 95 °C for 30 sec and 60 °C for 60 sec, followed by 72 °C for 1 min. All reactions were carried out in triplicate with a non-template control. The specific primers for p66Shc were as follows: forward, 5′-GCCGAGTATGTCGCCTA TGT-3′; reverse, 5′-GGGTGGGTTCCTGAGGTATT-3′. The primers for GADPH were as follows: forward, 5′-AGAAGGCTGGGGCTCATTTG-3′; reverse: 5′-AGGGGCCATCCACAGTCTTC-3′.
Western blotting assay
PBMs from DN patients (class IIa, class IIb, class III) and controls were prepared using radio immunoprecipitation assay (RIPA) buffer containing protease inhibitors. Protein concentration was measured with a Pierce® BCA Protein Assay Kit (Pierce Inc., USA). Thirty micrograms of total protein from each sample was subjected to electrophoresis and then transferred onto a PVDF membrane. The expression of p66Shc was assessed using a mouse monoclonal IgG anti-Shc/p66 (N-terminus) from LifeSpan BioSciences (1:1000 dilution). This antibody reacts with the p66Shc protein and not with the p46/p52 isoforms. The p-p66Shc protein expression was assessed using anti-Shc (phospho S36) (1:1000 dilution; Abcam). This antibody only recognizes the 66 kDa form of the Shc protein that is phosphorylated (p-p66Shc66 at Ser36), and it does not cross-react with unrelated phosphorylation sites in the protein or with the non-phosphorylated form of other Shc proteins. β–actin (1:6000 dilution) was used as an internal loading control. The ratio of p-p66Shc to actin and p66Shc to p-p66Shc was quantified using the Kodak 1D image analysis system.
Morphological analysis of kidneys
Four μm thick paraffin sections of a renal biopsy were stained with haematoxylin-eosin (H & E), periodic acid Schiff (PAS) and Masson. Tubular injury was scored as previously described19,45.
Immunohistochemistry
Renal biopsy tissue was paraffin-embedded and sectioned. After de-paraffinization, the sections were incubated with 3% H2O2 solution to block the endogenous peroxidase. Antigen retrieval was carried out using EDTA solution (pH 9.0) for 10 min. Sections were incubated with mouse monoclonal IgG anti-Shc/p66 (N-terminus) and anti-phospho-p66Shc (Ser36). They were incubated overnight at 4 °C, followed by incubation with horseradish peroxidase-conjugated secondary antibody and diaminobenzidine (DAB) substrate sequentially19,44,45. After haematoxylin counterstaining and dehydration, the sections were mounted and analysed with a Nikon microscope.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software. Data are expressed as the mean ± SD. Correlation analyses were carried out using Pearson’s correlation, Spearman’s correlation analysis and multiple regression analyses. p < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Xu, X. et al. p66Shc: A novel biomarker of tubular oxidative injury in patients with diabetic nephropathy. Sci. Rep. 6, 29302; doi: 10.1038/srep29302 (2016).
Change history
08 April 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Kanwar, Y. S., Sun, L., Xie, P., Liu, F. Y. & Chen, S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol 6, 395–423, 10.1146/annurev.pathol.4.110807.092150 (2011).
Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820, 10.1038/414813a (2001).
Zhu, K. et al. NADPH oxidase NOX1 is involved in activation of protein kinase C and premature senescence in early stage diabetic kidney. Free Radic Biol Med 83, 21–30, 10.1016/j.freeradbiomed.2015.02.009 (2015).
Zhan, M., Brooks, C., Liu, F., Sun, L. & Dong, Z. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int 83, 568–581, 10.1038/ki.2012.441 (2013).
Bock, F. et al. Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc. Proc Natl Acad Sci USA 110, 648–653, 10.1073/pnas.1218667110 (2013).
Trinei, M. et al. P66Shc signals to age. Aging (Albany NY) 1, 503–510 (2009).
Giorgio, M. et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122, 221–233, http://dx.doi.org/10.1016/j.cell.2005.05.011(2005).
Zhou, S. et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res 109, 639–648, 10.1161/CIRCRESAHA.111.243592 (2011).
Galimov, E. R. et al. P66shc action on resistance of colon carcinoma RKO cells to oxidative stress. Mol Biol (Mosk) 46, 139–146 (2012).
Park, Y. J. et al. p66Shc expression in proliferating thyroid cells is regulated by thyrotropin receptor signaling. Endocrinology 146, 2473–2480, http://dx.doi.org/10.1210/en.2004-1588 (2005).
Soliman, M. A. et al. The adaptor protein p66Shc inhibits mTOR-dependent anabolic metabolism. Sci Signal 7, ra17, 10.1126/scisignal.2004785 (2014).
Paneni, F. et al. Gene silencing of the mitochondrial adaptor p66(Shc) suppresses vascular hyperglycemic memory in diabetes. Circ Res 111, 278–289, 10.1161/CIRCRESAHA.112.266593 (2012).
Vashistha, H. et al. Null mutations at the p66 and bradykinin 2 receptor loci induce divergent phenotypes in the diabetic kidney. Am J Physiol Renal Physiol 303, F1629–F1640, 10.1152/ajprenal.00246.2012 (2012).
Yang, S. K., Xiao, L., Li, J., Liu, F. & Sun, L. Oxidative stress, a common molecular pathway for kidney disease: role of the redox enzyme p66Shc. Ren Fail 36, 313–320, 10.3109/0886022X.2013.846867 (2014).
Sun, L. et al. p66Shc mediates high-glucose and angiotensin II-induced oxidative stress renal tubular injury via mitochondrial-dependent apoptotic pathway. Am J Physiol Renal Physiol 299, F1014–F1025, 10.1152/ajprenal.00414.2010 (2012).
Ranieri, S. C. et al. Mammalian life-span determinant p66shcA mediates obesity-induced insulin resistance. Proc Natl Acad Sci USA 107, 13420–13425, 10.1073/pnas.1008647107(2010).
Menini, S. et al. Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes 55, 1642–1650, 10.2337/db05-1477 (2006).
Pagnin, E. et al. Diabetes induces p66shc gene expression in human peripheral blood mononuclear cells: relationship to oxidative stress. J Clin Endocrinol Metab 90, 1130–1136, http://dx.doi.org/10.1210/jc.2004-1283 (2005).
Sun, L. et al. Low-dose paclitaxel ameliorates fibrosis in the remnant kidney model by down-regulating miR-192. J Pathol 225, 364–377, 10.1002/path.2961 (2011).
Pan, H. Z. et al. The oxidative stress status in diabetes mellitus and diabetic nephropathy. Acta Diabetol 47 Suppl 1, 71–76, 10.1007/s00592-009-0128-1 (2010).
Al-Rubeaan, K. et al. Diabetic nephropathy and its risk factors in a society with a type 2 diabetes epidemic: a Saudi National Diabetes Registry-based study. PLoS One 9, e88956, 10.1371/journal.pone.0088956 (2014).
De Cosmo, S. et al. Kidney dysfunction and related cardiovascular risk factors among patients with type 2 diabetes. Nephrol Dial Transplant 29, 657–662, 10.1093/ndt/gft506 (2014).
Azeem, E. et al. Oxidative Stress Correlates (OSC) in Diabetes Mellitus Patients. Curr Diabetes Rev, CDR-EPUB-67484 (2015).
Krentz, A. J. Lipoprotein abnormalities and their consequences for patients with type 2 diabetes. Diabetes Obes Metab 5 Suppl 1, S19–S27 10.1046/j.1462-8902.2003.0310.x (2003).
Kawamura, M., Heinecke, J. W. & Chait, A. Pathophysiological concentrations of glucose promote oxidative modification of low density lipoprotein by a superoxide-dependent pathway. J Clin Invest 94, 771–778, 10.1172/JCI117396 (1994).
Kim, Y. R. et al. Epigenetic upregulation of p66shc mediates low-density lipoprotein cholesterol-induced endothelial cell dysfunction. Am J Physiol Heart Circ Physiol 303, H189–H196, 10.1152/ajpheart.01218.2011 (2012).
Shi, Y. et al. Oxidized low-density lipoprotein activates p66Shc via lectin-like oxidized low-density lipoprotein receptor-1, protein kinase C-beta, and c-Jun N-terminal kinase kinase in human endothelial cells. Arterioscler Thromb Vasc Biol 31, 2090–2097, 10.1161/ATVBAHA.111.229260 (2011).
Vallon, V. & Thomson, S. C. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol 74, 351–375, 10.1146/annurev-physiol-020911-153333 (2012).
Tang, S. C., Leung, J. C. & Lai, K. N. Diabetic tubulopathy: an emerging entity. Contrib Nephrol 170, 124–134, 10.1159/000325647 (2011).
Brezniceanu, M. L. et al. Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy. J Am Soc Nephrol 21, 943–954, 10.1681/ASN.2009030242 (2010).
Kim, C. S. et al. Homocysteine promotes human endothelial cell dysfunction via site-specific epigenetic regulation of p66shc. Cardiovasc Res 92, 466–475, 10.1093/cvr/cvr250 (2011).
Pani, G., Koch, O. R. & Galeotti, T. The p53-p66shc-Manganese Superoxide Dismutase (MnSOD) network: a mitochondrial intrigue to generate reactive oxygen species. Int J Biochem Cell Biol 41, 1002–1005, 10.1016/j.biocel.2008.10.011 (2009).
Kim, C. S. et al. p53 impairs endothelium-dependent vasomotor function through transcriptional upregulation of p66shc. Circ Res 103, 1441–1450, 10.1161/CIRCRESAHA.108.181644 (2008).
Orimo, M. et al. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol 29, 889–894, 10.1161/ATVBAHA.109.185694 (2009).
Deshpande, S. D. et al. Transforming growth factor-beta-induced cross talk between p53 and a microRNA in the pathogenesis of diabetic nephropathy. Diabetes 62, 3151–3162, 10.2337/db13-0305 (2013).
Chang, C. Y. et al. Taurine protects HK-2 cells from oxidized LDL-induced cytotoxicity via the ROS-mediated mitochondrial and p53-related apoptotic pathways. Toxicol Appl Pharmacol 279, 351–363, 10.1016/j.taap.2014.06.0299 (2014).
Zhou, L. et al. High glucose induces renal tubular epithelial injury via Sirt1/NF-kappaB/microR-29/Keap1 signal pathway. J Transl Med 13, 352, 10.1186/s12967-015-0710-y (2015).
Pinton, P. et al. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science 315, 659–663, 10.1126/science.1135380 (2007).
Efrati, S., Berman, S., Tov, Y. S., Averbukh, Z. & Weissgarten, J. Hyperglycemia alters renal cell responsiveness to pressure in a model of malignant hypertension. J Hypertens 27, 365–375, 10.1097/HJH.0b013e32831b46ab (2009).
Song, P. et al. PKCdelta promotes high glucose induced renal tubular oxidative damage via regulating activation and translocation of p66Shc. Oxid Med Cell Longev 2014, 746531, 10.1155/2014/746531 (2014).
Arany, I., Faisal, A., Nagamine, Y. & Safirstein, R. L. p66shc inhibits pro-survival epidermal growth factor receptor/ERK signaling during severe oxidative stress in mouse renal proximal tubule cells. J Biol Chem 283, 6110–6117, 10.1074/jbc.M708799200 (2008).
Foschi, M., Franchi, F., Han, J., La Villa, G. & Sorokin, A. Endothelin-1 induces serine phosphorylation of the adaptor protein p66Shc and its association with 14-3-3 protein in glomerular mesangial cells. J Biol Chem 276, 26640–26647, 10.1074/jbc.M102008200 (2001).
Tervaert, T. W. et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21, 556–563, 10.1681/ASN.2010010010 (2010).
Xiao, L. et al. Rap1 ameliorates renal tubular injury in diabetic nephropathy. Diabetes 63, 1366–1380, 10.2337/db13-1412 (2014).
Sun, L., Kondeti, V. K., Xie, P., Raparia, K. & Kanwar, Y. S. Epac1-mediated, high glucose-induced renal proximal tubular cells hypertrophy via the Akt/p21 pathway. Am J Pathol 179, 1706–1718, 10.1016/j.ajpath.2011.06.035 (2011).
Acknowledgements
This work was supported by grants from the Creative Research Group Fund of the National Foundation Committee of Natural Sciences of China (81470960, 81270812, 81570658, 81300600 and 81370832), Free Explore Plan of Central South University (2012QNZT146).
Author information
Authors and Affiliations
Contributions
X.Z. and M.M. generated the data for the manuscript and partially wrote the manuscript. X.X. generated the data for the manuscript. L.S. and X.Z. discussed the results of the manuscript. Y.H. and C.H. edited the manuscript. S.Y. and Y.Y. performed statistical analyses in the manuscript. L.X., F.L., Y.S.K. and L.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Xu, X., Zhu, X., Ma, M. et al. p66Shc: A novel biomarker of tubular oxidative injury in patients with diabetic nephropathy. Sci Rep 6, 29302 (2016). https://doi.org/10.1038/srep29302
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep29302
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.