Abstract
We seek to confirm the effect and explore the indications of aggressive locoregional management in patients with metastatic inflammatory breast cancer (IBC). Between 2003 and 2014, we reviewed the records of 156 patients with metastatic IBC from five large centers of Breast Surgery in the region of central south of China. Clinicopathologic data were collected to access overall survival (OS), prognostic factors and the indications for locoregional treatment. 75 (48%) patients underwent aggressive locoregional therapy. Patients in locoregional therapy group had a median OS of 24 months compared with 17 months of those in no locoregional therapy group. 2-year OS rate of these two groups was 52% and 32%, separately. Locoregional therapy (HR = 0.556; 95% CI 0.385–0.803; p = 0.002) was confirmed to be an independent prognostic factor, which could significantly improve OS of patients with metastatic IBC. For locoregional therapy group, statistical differences were observed in all subgroups stratified by the factors that were significant in univariate analysis except in the subgroups of stable disease, Charlson comorbidity index ≥3 and cerebral metastasis. Therefore, systemic therapy efficacy, Charlson comorbidity index and cerebral metastasis status appeared to be important indexes for choice of locoregional therapy in different individuals.
Similar content being viewed by others
Introduction
Breast cancer has become the leading cancer among women worldwide, which accounts for 25% of female cancer1. Due to advanced diagnostic technology and effective multimodality therapy, long term survival of early breast cancer shows great improvement. According to the data from National Cancer Center of China in 2015, the estimated 5-year prevalence for women breast cancer was 1.02 million2. Based on this, our research focus has gradually turned to refractory breast cancer.
Inflammatory breast cancer (IBC) is the most fatal form of breast cancer, which accounts for only 1–6% of all breast tumors but roughly 10% of breast cancer motality annually, with an elevated incidence3,4,5. Lacking of specific histological and molecular subtype, the diagnosis of IBC relies mainly on clinical features as follows6: rapid and progressive onset of breast erythema, edema or peau d’orange occupying at least one third of the breast (with or without an underlying mass); maximum sympotomatic duration of 6 months; pathologic confirmation of invasive carcinoma.
Due to its aggressive nature, the prognosis of IBC is extremely poor. Multiple recent studies have reported 24–50% metastasis rate and 34–64% 5-year overall survival (OS) rate in IBC patients7,8,9,10,11. For metastatic IBC, 2-year OS and 5-year OS is 39% and 29–33% respectively7,12,13. Since several important agents such as paclitaxel, docetaxel and trastuzumab were approved for the therapy of breast cancer (BC), and trimodality treatment (anthracycline-based neoadjuvant chemotherapy, modified radical mastectomy, postmastectomy radiation therapy) has gained wide acceptence, the survival of BC patients has obtained great improvement. IBC also benefit from these advances14.
For common metastatic BC, aggressive locoregional management were proved to be effective in some retrospective non-randomized clinical studies15,16,17,18, such as complete excision of primary breast tumor and radiation therapy to the chest wall and draining lymphatics. Based on these findings, several prospective randomized trials are ongoing. With regard to metastatic IBC, studies are still fairly limited because of less proportion of patients. A recent study confirmed effectivity of primary tumor resection in metastatic IBC13.
For this study, we not only analyzed the role of locoregional therapy in metastatic IBC, but also explored the indications for selecting patients who could benefit from this approach. The factors influencing prognosis of metastatic IBC were also researched.
Methods
Data Source
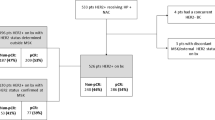
Between 2003–2014, we collected 156 metastatic IBC patients’ clinicopathologic data in the region of central south of China from five large centers of Breast Surgery (taxane and trastuzumab were widely used for breast cancer in this developing country since 2003). Multimodality treatment regimens were based on chemotherapy including anthracycline and taxane. Trastuzumab and endocrinotherapy were used in part of patients in line with the indication.
Ethics approval
The research was reviewed and approved by the Ethics Committee of the Xiangya Hospital of Central South University and was conducted from June 2003 to June 2014. This is to certificate that the research design and methods are in accordance with the requirements of regulations and procedures regarding to human subject protection laws such as GCP and ICH-GCP. This study is a retrospective study without any type of clinical intervention.
Diagnostic criteria
The diagnostic criteria of IBC include sudden onset of inflammation expression of over 1/3 of the range of unilateral breast skin, less than 6 months of duration, and pathologically confirmed as invasive breast cancer6. The AJCC Cancer Staging Manual was used to identify TNM-staging19. Patients were included only if metastases were found less than 3 months from the diagnosis of IBC.
Clinicopathological features assessment
Patient’s clinicopathologic features were collected from records, including age, histological classification, menstrual status, Charlson comorbidity index (graded as ≤2 and ≥3), lymph node involvement, status of estrogen receptor (ER)/progestrone receptor (PgR), status of human epidermal growth factor receptor 2 (HER-2), number and site of metastasis, therapeutic regimens, evaluation of clinical response to systemic therapy (graded as complete remission; partial remission; stable disease). Lymph node involvement and clinical response to systemic therapy were assessed by physical examination and imaging examination. Clinical response of no locoregional therapy group was assessed by the result of the last period of systemic therapy. Surgery in this study was for treatment rather than palliative. The endpoint of this study was IBC-related death or the last follow-up date.
Statistical Analysis
The Kaplan-Meier method was applied to evaluate survival of patients. We used log-rank test to compare survival curves between groups, and stratification analysis to compare survival benefit of locoregional therapy for different subgroups. The patients’ characteristics and associations between groups were analyzed with the Pearson’s Chi squared test. Prognostic factors with p < 0.10 using univariate analysis were entered into the multivariate analysis model. Cox proportional hazards models were fit to assess relationship of these factors and determine independent prognostic factor. Hazard ratios (HRs) and 95% CIs were also recorded. The α-level of 5% was used to determine statistical significance. All tests were two-sided and all statistical analyses were performed by Statistical Package for Social Sciences version 19.
Results
Clinicopathologic features of the whole cohort
Among 156 patients, 13 (8%) cases refused to receive any kind of therapy after their diagnosis because of psychological and financial reasons. The rest 143 patients received tanxane and anthracycline-based chemotherapy. 64 (41%) patients presented HER-2 positive and only 21 (33%) of them accepted the treatment of trastuzumab for financial reasons. 70 (45%) of 156 patients demonstrated hormone receptor positive, 61 (87%) of them received endocrine therapy. For locoregional therapy group, 52 (33%) underwent surgical resection of the primary tumor and axillary lymph node. 11 patients of them underwent metastasectomy in the meantime. The time from diagnosis to surgery ranged from 2–15 months. All of them were given adjuvant/neoadjuvant radiation therapy to chest wall and draining nodal volumes. 23 (15%) patients only received locoregional radiation therapy. Among 75 patients treated with radiation therapy, 37 (49%) were delivered a median of 50 Gy in twice-daily fractions of 2 Gy, 15 (20%) patients were delivered a median of 54 Gy in once-daily fractions of 2 Gy, 23 (31%) patients’ regimens were unknown. There were another 21 patients received locoregional symptomatic therapy. Biological agents (pseudomonas aeruginosa injection) were given to 5 patients and interventional embolization were given to 8 patients. The rest 8 patients underwent other forms of palliative surgery. These patients were excluded from locoregional therapy group. Patient characteristics for locoregional therapy group and no locoregional therapy group were summarized in Table 1.
The factors may lead to selection bias
Patients who underwent locoregional therapy were more likely to have negative Her-2 receptor (67% vs 52%, P = 0.060) and positive locoregional lymph nodes (91% vs 80%, P = 0.067). They were also more inclined to have single metastasis loci than patients who didn’t receive locoregional therapy (76% vs 63%, P = 0.078). Only 6 (8%) patients with cerebral metastasis underwent locoregional therapy, compared with 18 (22%) patients in no locoregional therapy group (P = 0.014). This trend was reversed in patients with soft tissue metastasis (28% vs 16%, P = 0.071).
Prognosis for locoregional therapy group and matched group
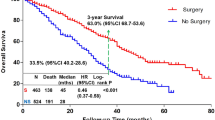
The median OS of all patients was 21 months. 2-year OS and 3-year OS for the entire cohort was 42% and 29% separately. Patients in locoregional therapy group had a median overall survival of 24 months compared with 17 months of those in no locoregional therapy group (p < 0.001, Log-Rank Test). 2-year OS of two groups was 52% and 32% separately. With the exclusive of patients who didn’t receive Herceptin, 21 of 64 cases accepted the treatment of Herceptin. 11 of 21 patients received locoregional therapy. The median OS of this cohort was 28 months. The median OS are 33 months and 15 months separately for locoregional therapy group and no locoregional therapy group (P = 0.055, Log-Rank Test). According to different regimens, patients were divided into 4 groups (surgery + radiation therapy + systemic therapy; radiation therapy + systemic therapy; systemic therapy alone and no therapy). The median OS of four groups was 26 months, 19 months, 18 months and 13 months (p < 0.001, Log-Rank Test).
The results in univariate analysis
In univariate analysis, a few factors that were significantly associate with OS were as follows: histological grade, Charlson comorbidity index, hormone receptor, Her-2 receptor, systemic therapy efficacy, osseous metastasis, cerebral metastasis and soft tissue metastasis. Other facors, such as locoregional lymph nodes status, number of metastatic sites, visceral metastasis, didn’t significantly influence the prognosis (Table 2).
The results in multivariate analysis
Prognostic factors with p < 0.10 using univariate analysis entered into the multivariate analysis model. After adjustment of all relevant covariates, locoregional therapy (HR = 0.556; 95%CI 0.385–0.803; p = 0.002) was confirmed to be an independent prognostic factor associated with OS (Fig. 1A). For the purpose of further research in specific locoregional regimens, another multivariable cox regression model was introduced (Table 3). Compared with no therapy group, surgery + radiation therapy + systemic therapy (HR = 0.219; 95%CI 0.090–0.529; p = 0.001), radiation therapy + systemic therapy (HR = 0.362; 95%CI 0.148–0.885; p = 0.026) and systemic therapy alone (HR = 0.438; 95%CI 0.190–1.012; p = 0.053) were associated with a better survival (Fig. 1B). Beside locoregional therapy, other factors such as positive hormone receptor, systemic therapy with CR, osseous metastasis, soft tissue metastasis appeared to be independent prognostic factors for better survival. On the contrary, cerebral metastasis was identified to be associate with a worse survival (Table 3).
The indications for locoregional therapy in metastatic IBC
In stratified analysis, the prognostic factors with statistical significance in univariate analyses were divided into subgroups based on different states. For each subgroup, OS was analyzed by Kaplan–Meier method according to locoregional therapy. Finally, we observed statistical differences of OS in patients with CR (Fig. 2A, 62 months vs 25 months, p = 0.005) or PR (Fig. 2B, 22 months vs 18 months, p = 0.002) or without cerebral metastasis (Fig. 3A, 25 months vs 19 months, p < 0.001) between locoregional therapy group and no locoregional therapy group. Patients with SD (Fig. 2C, 18 months vs 15 months, p = 0.534) or with cerebral metastasis (Fig. 3B, 12 months vs 13 months, p = 0.934) didn’t benefit from locoregional therapy. Besides, 2 points of Charlson comorbidity index was defined as a cut-off point. For patients with Charlson comorbidity index ≤2, locoregional therapy could significantly improve OS compared with no locoregional therapy group (Fig. 4A, 31 months vs 17 months, p < 0.001). However, statistical difference wasn’t observed in patients with Charlson comorbidity index ≥3 between these two groups (Fig. 4B, 21 months vs 18 months, p = 0.102).
(A) locoregional therapy versus no locoregional therapy in patients with CR (Median OS: 62 months vs 25 months; p = 0.005). (B) locoregional therapy versus no locoregional therapy in patients with PR (Median OS: 22 months vs 18 months; p = 0.002). (C) locoregional therapy versus no locoregional therapy in patients with SD (Median OS: 18 months vs 15 months; p = 0.534).
(A) locoregional therapy versus no locoregional therapy in patients with Charlson comorbidity index ≤2 (Median OS: 31 months vs 17 months; p < 0.001). (B) locoregional therapy versus no locoregional therapy in patients with Charlson comorbidity index ≥3 (Median OS: 21 months vs 18 months; p = 0.102).
Discussion
This retrospective multicentre research analysed the effect of aggressive locoregional management and explore the indications for this approach in metastatic IBC. In order to exclude the impact of different therapeutic regimens in different decades, we collected 156 patients diagnosed after 2003 when drugs like taxane and trastuzumab and multimodality treatment were widely used for breast cancer in this developing country. 75 (48%) patients in this study underwent non-palliative locoregional therapy, while the proportion were 44% and 56% in two related studies recently10,13. Among these patients, 69%, 58% and 70% of them received both surgery and postmastectomy radiation in our study and these two related analyses separately. This trend is significantly different from earlier studies for common metastatic BC. Lang et al.18 and Ruiterkamp et al.20 reported respectively that 24 of 90 (27%) and 256 of 634 (40%) patents with locoregional therapy received both surgery and postmastectomy radiation. With regard to more earlier studies, postmastectomy radiation played a relatively nonsignificant role in common metastatic BC15,21. They even didn’t mention postmastectomy radiation as a prognostic factor in their studies. But now things have changed, and trimodality treatment is confirmed to improve survival of patients with BC and IBC6,20. Surgery or radiation therapy alone is increasingly rare, and integrated locoregional regimens are recommended. Therefore, in our research, we made a comprehensive analysis of surgery and postmastectomy radiation rather than surgery alone.
In this study, we found that aggressive locoregional management could improve OS of patients with metastatic IBC in both univariate analysis and multivariate analysis. The results reported 24 months of median OS and 52% of 2-year OS rate in patients with aggressive locoregional management, compared with 17 months and 32% in patients who didn’t receive this approach. Warren et al.10 revealed in their research a median OS of 2 years for patients with metastatic IBC who received locoregional control, which was similar to our results. Akay et al.13 reported that primary tumor resection was associated with a nearly 5-fold increase in OS, which showed a stronger effect of locoregional therapy on stage IV IBC. In our analysis, the hazard ration of locoregional therapy group was 0.556 compared to no locoregional therapy group after adjustment of relevant covariates. In contrast with radiation therapy alone, surgical resection of primary tumor combined with postmastectomy radiation therapy were significantly associated with better survival (HR 0.219, 95%CI 0.090–0.529 vs HR 0.362, 95%CI 0.148-0.885). This result was also consistent with the data in Akay’s study mentioned before13.
In this analysis, we first explored the indications for locoregional management in metastatic IBC, looking for clinicopathological indexes of patients which suggested benefit from this regimen. Finally, we filtered out three variates as the indexes for selection of locoregional management from significant variates in univariate analysis, which were systemic therapy efficacy, Charlson comorbidity index, and cerebral metastasis status. Systemic therapy could improve OS of patients with metastatic IBC according to our study, which was also proved in other research about stage IV IBC13 and BC21,22. After making a further comparative study, we found that not all the patients with locoregional management could benefit from systemic therapy. Only in patients with CR or PR, locoregional management could bring them better OS. Cerebral metastasis was considerd to have a great impact on prognosis, with the OS ranging from 5 months to 13 months23,24. In our research, patients with cerebral metastasis couldn’t benefit from locoregional management. We suggest that aggressive locoregional management should be carefully evaluated for these patients. Charlson comorbidity index was first proposed in 198725. The index has gained wide acceptance as a important prognostic parameter. By repeated investigation, we found a cut-off point. When Charlson comorbidity index was no more or more than 2 points, patients could benefit or not benefit from locoregional management. Therefore, for patients with several coexisting illnesses (≥3), we also need to think carefully about the necessity of aggressive locoregional management.
Due to lack of relevant prospective randomized trial, the results of current studies on common metastatic BC were limited by selection bias. Several studies showed that some factors such as primary tumor burden, number of metastatic sites, location of metastasis, response to systemic therapy, performance status and comorbidity index might lead to selection bias18,26,27. Because these factors were often associated with survival, survival benefit of surgery may be a weak argument. A study by Leung et al. demonstrated that after adjustment for administration of chemotherapy, no statistical difference was seen between surgery group and no surgery group28. Another study also suggested that most of the survival advantage could attribute to selection bias29. In our study, we analysed demographic and covariate information of locoregional therapy group and no locoregional therapy group (Table 1). Patients of locoregional therapy group were less likely to have more than one metastasis (P = 0.078), and more likely to have soft tissue metastasis (P = 0.071) and negative Her-2 receptor (P = 0.060). However, these differences didn’t reach statistical significance. Cerebral metastasis between two groups was significantly different (P = 0.014). These factors were analysed in multivariate analysis model. After adjustment of influence by these factors, survival advantage was still seen in locoregional group (Fig. 1A). Because lymph nodes involvement didn’t affect survival in univariate analysis, the variate didn’t entered into multivariate analysis model.
Conclusion
Aggressive locoregional treatment could significantly improve OS of patients with metastatic IBC. Systemic therapy efficacy, Charlson comorbidity index and cerebral metastasis status appeared to be important indexes for choice of locoregional therapy in different individuals.
Additional Information
How to cite this article: Yan, Y. et al. The role and indications of aggressive locoregional therapy in metastatic inflammatory breast cancer. Sci. Rep. 6, 25874; doi: 10.1038/srep25874 (2016).
References
Torre, L. A. et al. Global cancer statistics, 2012. CA: a cancer journal for clinicians 65, 87–108, doi: 10.3322/caac.21262 (2015).
Zheng, R., Zeng, H., Zhang, S., Chen, T. & Chen, W. National estimates of cancer prevalence in China, 2011. Cancer letters, doi: 10.1016/j.canlet.2015.10.003 (2015).
Levine, P. H. & Veneroso, C. The epidemiology of inflammatory breast cancer. Seminars in oncology 35, 11–16, doi: 10.1053/j.seminoncol.2007.11.018 (2008).
Walshe, J. M. & Swain, S. M. Clinical aspects of inflammatory breast cancer. Breast disease 22, 35–44 (2005).
Woodward, W. A. Inflammatory breast cancer: unique biological and therapeutic considerations. The Lancet. Oncology 16, e568–576, doi: 10.1016/S1470-2045(15)00146-1 (2015).
Dawood, S. et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 22, 515–523, doi: 10.1093/annonc/mdq345 (2011).
Cristofanilli, M. et al. Inflammatory breast cancer (IBC) and patterns of recurrence: understanding the biology of a unique disease. Cancer 110, 1436–1444, doi: 10.1002/cncr.22927 (2007).
Smoot, R. L. et al. A single-center experience with inflammatory breast cancer, 1985–2003. Archives of surgery 141, 567–572 discussion 572-563, doi: 10.1001/archsurg.141.6.567 (2006).
Brown, L. et al. Once-daily radiation therapy for inflammatory breast cancer. International journal of radiation oncology, biology, physics 89, 997–1003, doi: 10.1016/j.ijrobp.2014.01.054 (2014).
Warren, L. E. et al. Inflammatory Breast Cancer: Patterns of Failure and the Case for Aggressive Locoregional Management. Annals of surgical oncology 22, 2483–2491, doi: 10.1245/s10434-015-4469-4 (2015).
Mego, M. et al. Circulating tumor cells in newly diagnosed inflammatory breast cancer. Breast cancer research: BCR 17, 2, doi: 10.1186/s13058-014-0507-6 (2015).
Dawood, S. et al. Identifying factors that impact survival among women with inflammatory breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO 23, 870–875, doi: 10.1093/annonc/mdr319 (2012).
Akay, C. L. et al. Primary tumor resection as a component of multimodality treatment may improve local control and survival in patients with stage IV inflammatory breast cancer. Cancer 120, 1319–1328, doi: 10.1002/cncr.28550 (2014).
Dawood, S. et al. Survival of women with inflammatory breast cancer: a large population-based study. Annals of oncology official journal of the European Society for Medical Oncology/ESMO 25, 1143–1151, doi: 10.1093/annonc/mdu121 (2014).
Khan, S. A., Stewart, A. K. & Morrow, M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery 132, 620–626 discussion 626-627 (2002).
Gnerlich, J. et al. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988-2003 SEER data. Annals of surgical oncology 14, 2187–2194, doi: 10.1245/s10434-007-9438-0 (2007).
Nguyen, D. H. et al. Can locoregional treatment of the primary tumor improve outcomes for women with stage IV breast cancer at diagnosis? International journal of radiation oncology, biology, physics 84, 39–45, doi: 10.1016/j.ijrobp.2011.11.046 (2012).
Lang, J. E. et al. Primary tumor extirpation in breast cancer patients who present with stage IV disease is associated with improved survival. Annals of surgical oncology 20, 1893–1899, doi: 10.1245/s10434-012-2844-y (2013).
Singletary, S. E. & Connolly, J. L. Breast cancer staging: working with the sixth edition of the AJCC Cancer Staging Manual. CA: a cancer journal for clinicians 56, 37–47 quiz 50-31 (2006).
Liauw, S. L., Benda, R. K., Morris, C. G. & Mendenhall, N. P. Inflammatory breast carcinoma: outcomes with trimodality therapy for nonmetastatic disease. Cancer 100, 920–928, doi: 10.1002/cncr.20083 (2004).
Chia, S. K. et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 110, 973–979, doi: 10.1002/cncr.22867 (2007).
Ernst, M. F. et al. Trends in the prognosis of patients with primary metastatic breast cancer diagnosed between 1975 and 2002. Breast 16, 344–351, doi: 10.1016/j.breast.2007.01.001 (2007).
Ho, V. K. et al. Survival of breast cancer patients with synchronous or metachronous central nervous system metastases. European journal of cancer, doi: 10.1016/j.ejca.2015.07.040 (2015).
Bendell, J. C. et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97, 2972–2977, doi: 10.1002/cncr.11436 (2003).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases 40, 373–383 (1987).
Fields, R. C. et al. Surgical resection of the primary tumor is associated with increased long-term survival in patients with stage IV breast cancer after controlling for site of metastasis. Annals of surgical oncology 14, 3345–3351, doi: 10.1245/s10434-007-9527-0 (2007).
Ruiterkamp, J. et al. Surgical resection of the primary tumour is associated with improved survival in patients with distant metastatic breast cancer at diagnosis. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 35, 1146–1151, doi: 10.1016/j.ejso.2009.03.012 (2009).
Leung, A. M., Vu, H. N., Nguyen, K. A., Thacker, L. R. & Bear, H. D. Effects of surgical excision on survival of patients with stage IV breast cancer. The Journal of surgical research 161, 83–88, doi: 10.1016/j.jss.2008.12.030 (2010).
Cady, B., Nathan, N. R., Michaelson, J. S., Golshan, M. & Smith, B. L. Matched pair analyses of stage IV breast cancer with or without resection of primary breast site. Annals of surgical oncology 15, 3384–3395, doi: 10.1245/s10434-008-0085-x (2008).
Acknowledgements
For their assistance in providing clinical cases from electronic medical record systems, the authors gratefully acknowledge the following departments and fellows, the Department of Breast Surgery (Jian Hai), Xiangya Hospital; the Department of Breast and Thyroid Surgery (Fenfen Fu), the Sencond Xiangya Hospital; the Department of Gastroenterology (Ai Feiyan), the Third Xiangya Hospital, Central South University; the Department of Breast and Thyroid Surgery (Chaojie Zhang), Hunan Provincial People’s Hospital; and the Department of Breast Surgery (Zhaoyun Wu), Hunan Provincial Tumor Hospital.
Author information
Authors and Affiliations
Contributions
Y.Y. sorted and analyzed the clinicopathological data collected by other authors, strictly selected cases according to the research requirements, designed the methods used in the study and writed the manuscript. L.T. accessed the feasibility and innovation of the study, offered the help to cooperate with multiple research centers, and collected the medical records needed by the study. W.T. gave assistance to first author to analyze and interpret research data, helped to design figures and tables and collected the medical records needed by the study. J.Z. summarized all preliminary data collected by all authors and other research workers, obtained follow-up information of all cases, collected quite a few of the medical records needed by the study and gave guidance to designing, writing and revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yan, Y., Tang, L., Tong, W. et al. The role and indications of aggressive locoregional therapy in metastatic inflammatory breast cancer. Sci Rep 6, 25874 (2016). https://doi.org/10.1038/srep25874
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep25874
This article is cited by
-
Clinical outcomes of de novo metastatic HER2-positive inflammatory breast cancer
npj Breast Cancer (2023)
-
Tailoring Treatment for Patients with Inflammatory Breast Cancer
Current Treatment Options in Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.