Abstract
To examine the influence of elevated temperature and nitrogen (N) addition on species composition and development of arbuscular mycorrhizal fungi (AMF) and the effect of AMF on plant community structure and aboveground productivity, we conducted a 5-year field experiment in a temperate meadow in northeast China and a subsequent greenhouse experiment. In the field experiment, N addition reduced spore population diversity and richness of AMF and suppressed the spore density and the hyphal length density (HLD). Elevated temperature decreased spore density and diameter and increased the HLD, but did not affect AMF spore population composition. In the greenhouse experiment, AMF altered plant community composition and increased total aboveground biomass in both elevated temperature and N addition treatments; additionally, AMF also increased the relative abundance and aboveground biomass of the grasses Leymus chinensis (Poaceae) and Setaria viridis (Gramineae) and significantly reduced the relative abundance and aboveground biomass of the Suaeda corniculata (Chenopodiaceae). Although elevated temperature and N addition can affect species composition or suppress the development of AMF, AMF are likely to play a vital role in increasing plant diversity and productivity. Notably, AMF might reduce the threat of climate change induced degradation of temperate meadow ecosystems.
Similar content being viewed by others
Introduction
Arbuscular mycorrhizal fungi (AMF) play a vital role in the survival and development of plants in terrestrial ecosystems by improving growth and increasing productivity1,2,3, which determines ecosystem stability and multifunctionality4,5,6. Moreover, AMF influence many other ecosystem processes7, including carbon (C) cycling by accelerating the decomposition of organic matter7,8,9 and nitrogen (N) cycling by improving the uptake and transfer of nitrogen10,11 and by reducing nitrogen leach12, among other processes. However, the species composition and the development of AMF are also influenced by changes in climate, such as elevated temperature and nitrogen addition, although the ecological determinants that regulate these parameters of AMF are not well understood.
Global climate change induced by human activities influences the development and species composition of AMF. Elevated temperature increases the allocation of carbon to AMF and hyphae length13,14 and improves mycorrhizal colonization of root systems15,16. However, in several studies, elevated temperature had no effect on the length of roots colonized, spore density or the extraradical hyphal density of AMF13,17. Elevated temperature not only influences the development of AMF in soil and roots but also alters the species composition. The relative abundance and function of AMF are influenced by the variation of seasons and temperatures18 and the effect of elevated temperature on the species richness of AMF in soil can be positive19 or have no effect on AMF species composition17. These observations of varying responses suggest that the effects of elevated temperature on AMF are not uniform. For instance, warming increased AMF species richness in a semiarid steppe ecosystem19 in northern China, while warming did not affect AMF species composition in a native grassland in UK17 and Qinghai Tibet Plateau in China20.
As one of the major threats to ecosystem stability, nitrogen (N) deposition reduces plant species number21. Moreover, N inputs also have an important effect on species composition in the AMF community22. N fertilization significantly decreased AMF spore abundance, but no impact on spore species richness and AM hyphal density in a field experiment at the Cedar Creek Ecosystem Science Reserve, MN, USA23. The result from an alpine meadow ecosystem of China found that N fertilization reduced the abundance of Glomeromycota and AMF species richness24. In many studies, a reduction in the length of root colonization has been observed23,24, but other studies have reported an increase in the AMF colonization of roots after N fertilization25. Although the separate effects of elevated temperature and N addition on the AM fungal community have received considerable attention15,23,24, global elevated temperature and N deposition frequently occur simultaneously. Elevated temperature frequently enhances soil microbial activity and increase N mineralization26. Although limited, the effects of combined elevated temperature and nitrogen fertilization on soil microorganisms have been examined27,28,29. Nevertheless, the integrated effects of elevated temperature and N addition on AM fungal communities in temperate meadow ecosystems in China are not well understood.
Many studies demonstrated that plant-induced alterations of the soil affect the structure of the plant community30,31,32. Negative plant-soil feedback plays an important role in maintaining plant community diversity33,34,35, whereas positive plant-soil feedback may reduce plant diversity36,37. However, it is unclear which type of plant-soil feedback will occur during climate change.
The Songnen grassland is located at the eastern edge of the Eurasian grassland and is the largest and most typical meadow steppe in China38. The average temperature of the Songnen meadow steppe has increased by 2 °C in the last twenty years39. The average atmospheric N deposition is approximately 10.5 g m−2 yr−1.40 Therefore, to study the effects of elevated temperature and N addition on plant community composition and productivity, a field experiment was established in 2006 in the Songnen meadow ecosystem41. However, the responses of AMF to elevated temperature and N addition and the effects of changes in AMF on the plant community composition and productivity are not clear in the Songnen meadow ecosystem in northeastern China.
To better understand the effects of elevated temperature and N addition on the development and species community composition of AMF communities and their feedbacks on the aboveground plant community, a 5-year elevated temperature and N addition field experiment and a greenhouse experiment were conducted. We hypothesized that (1) N addition and elevated temperature would affect the development and species composition of AMF in the field experiment and that (2) AMF would alter the composition of the plant community and increase plant diversity and productivity under the elevated temperature and N addition conditions in the greenhouse experiment because of AMF can reduce the negative of N addition and elevated temperature on plant growth.
Results
Spore development and species composition of AMF in the field experiment
The elevated temperature treatment decreased the AMF spore density and diameter by 32% (P < 0.05) and 10% (P < 0.05), respectively and increased the hyphal length density (HLD) by 27% (P < 0.001). The N addition treatment decreased the AMF HLD 14% (P < 0.05, Table 1). Significant interactive effects of elevated temperature × N addition on AMF spore density and diameter were detected (all P < 0.01), but no interactive effect on HLD was observed (P > 0.05; Table 1).
The species composition of AMF community was significantly affected by the elevated temperature and N addition treatments. Elevated temperature had no impact on AMF species richness, diversity (H) or evenness (E) (all P > 0.05; Table 1), whereas N addition decreased AMF species richness and H by 18% (P < 0.05) and 25% (P < 0.05), respectively, compared with control, but had no impact on E (Table 1). No interactive effects of elevated temperature × N addition on H and E were detected (all P > 0.05), but a significant interactive effect of elevated temperature × N addition on species richness was observed (P < 0.05).
Plant community composition in the greenhouse experiment
AMF significantly increased plant diversity (Fig. 1a), richness (Fig. 1b) and evenness (Fig. 1c) both in elevated temperature and N addition treatments. In elevated temperature treatment, AMF significantly increased plant species diversity, richness and evenness, the mycorrhizal benefits of them were 17.1, 11.1 and 13.6, respectively (all P > 0.05). In N addition treatment, AMF significantly altered plant community composition, the mycorrhizal benefits of plant diversity, richness and evenness were 16.3 33.8 and 11.7, respectively and significant differences between AM and NM were observed (all P > 0.05). Compared to control, the mycorrhizal benefits on plant diversity, richness and evenness all significantly increased (all P < 0.05) both in elevated temperature and N addition treatments. Significant interactive effects of N addition × AMF on plant diversity, richness and evenness were observed and significant interactive of elevated temperature × AMF on plant diversity and evenness were also observed. No interactive effects of elevated temperature × N addition × AMF on plant species diversity, richness and evenness were detected (Table 2).
Effects of AMF on plant diversity (a), richness (b) and evenness (c) under elevated temperature and N addition in the greenhouse experiment. The lowercase letters in each column represent significant difference (P < 0.05) among different treatments in the same AMF treatment. Asterisks represent significant difference (P < 0.05) between with and without AMF treatments in the same N, T or T + N treatments.
AMF altered plant species composition both in elevated temperature and N addition treatments (Fig. 2). In elevated temperature, AMF significantly increased the abundance of L. chinensis, L. davurica and C. virgata, the mycorrhizal benefits of them were 32.5, 29.6 and 11.1 (all P < 0.01), respectively; but the abundance of S. viridis and S. corniculata significantly decreased by AMF and the mycorrhizal benefits were −9.6 (P < 0.05) and −28.6 (P < 0.05). In N addition treatment, AMF had no impact on the abundance of S. viridis (P > 0.05) and C. virgata (P > 0.05) and AMF decreased abundance of S. corniculata (P < 0.05) and the mycorrhizal benefit was −20.3 whereas AMF increased the abundance of L. chinensis and L. davurica and the mycorrhizal benefits of them were 25.0 (P < 0.05) and 16.7 (P < 0.05). Significant interactive effects of N addition × AMF on the abundances of L. chinensis and S. corniculata were observed, no significant interactive effects of elevated temperature × N addition, elevated temperature × AMF and elevated temperature × N addition × AMF were detected (Table 3).
Species density composition (%) of total number of individuals in four control (a,b), N addition (c,d), elevated temperature (e,f) and elevated temperature plus N addition treatments (g,h) with AMF (AM) or without AMF (NM) in the greenhouse experiment. Species included are (clockwise from top) L. chinensis (oblique line); S. viridis (dots); L. davurica (grey); C. virgata (black); S. corniculata (clear). Data represent the mean of six replicates.
Aboveground plant biomass in the greenhouse experiment
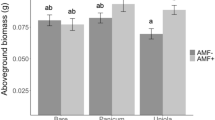
In control, AMF increased aboveground biomass of L. chinensis and S. viridis (Fig. 3) and the mycorrhizal benefits were 240.6 (P < 0.01) and 258.5 (P < 0.01), respectively, but significantly decreased the biomass of C. virgata (P < 0.05) and S. corniculata (P < 0.05). In elevated temperature treatment, aboveground biomass of L. chinensis, S. viridis, L. davurica significantly increased by AMF, the mycorrhizal benefits of them were 366.0 (P < 0.01), 374.4 (P < 0.01) and 57.9 (P < 0.05), respectively, AMF decreased aboveground biomass of C. virgata and S. corniculata significantly. In the N addition treatment, AMF increased aboveground biomass of L. chinensis, S. viridis and L. davurica, the mycorrhizal benefits were 51.1, 47.4 and 186.8, respectively, whereas the aboveground biomass of S. corniculata decreased significantly. No significant interactive effects of elevated temperature × N addition, N addition × AMF, or elevated temperature × N addition × AMF on the aboveground biomass of L. davurica and C. virgata were detected (Table 3). Significant main effects of elevated temperature, N addition, AMF and interactive effects of elevated temperature × N addition and elevated temperature × N addition × AMF on the aboveground biomass of L. chinensis and S. corniculata were detected (Table 3). Moreover, AMF significantly increased total aboveground biomass both in elevated temperature and N addition treatments (Fig. 3f).
The effects of AMF on plant biomass under N addition and elevated temperature in the greenhouse experiment.
(a) L. chinensis; (b) S. viridis; (c) L. davurica; (d) C. virgata; (e) S. corniculata; (f) Total aboveground biomass. The lowercase letters in each column represent significant difference (P < 0.05) among different treatments in the same AMF treatment. Asterisks represent significant difference (P < 0.05) between with and without AMF treatments in the same N, T or T + N treatments.
Plant biomass composition in the greenhouse experiment
AMF altered the relative contribution of each plant species to the total aboveground biomass in elevated temperature and N addition treatments (Fig. 4). In elevated temperature treatment, AMF significantly increased the proportion of total plant from L. chinensis and S. viridis, the mycorrhizal benefits were 135.4 (P < 0.01) and 124.8 (P < 0.01), respectively, whereas decreased the proportion of total plant from C. virgata and S. corniculata and the mycorrhizal benefits were −13.7 (P < 0.05) and −77.2 (P < 0.05), respectively. Compared to control, the mycorrhizal benefits of L. chinensis, S. viridis and L. davurica significantly increased, but the mycorrhizal benefits of S. corniculata decreased. In the N addition treatment, AMF also increased the proportion of total plant from L. chinensis and S. viridis, the mycorrhizal benefits were 133.7 (P < 0.01) and 259.5 (P < 0.01), whereas decreased the proportion of total plant from L. davurica and S. corniculata and the mycorrhizal benefits were −35.7 (P < 0.05) and −78.4 (P < 0.05), respectively. The mycorrhizal benefits of L. chinensis, S. viridis and L. davurica in elevated temperature and N addition treatments significantly increased (all P < 0.05) compared to control, but the mycorrhizal benefits of S. corniculata decreased, significantly. Additionally, significant main effects of AMF and interactive effects of AMF × N addition on the proportions of L. chinensis and S. corniculata to total biomass were observed (Table 3).
Species composition (%) of total aboveground biomass in four control (a,b), N addition (c,d), elevated temperature (e,f) and elevated temperature plus N addition treatments (g,h) in pots with AMF (AM) or without AMF (NM). Species included are (clockwise from top) L. chinensis (oblique line); S. viridis (dots); L. davurica (grey); C. virgata (black); S. corniculata (clear). Data represent the mean of six replicates.
Discussion
Based on our results, changes in climate (elevated temperature and N addition) affected the development of AMF and determined the composition of AMF spores population. Although the effect of elevated temperature on the composition of the AMF has been examined in many field studies of different ecosystems20,28,42, the response of the AMF was not consistent in these studies. In the temperate steppe (Inner Mongolia, China), elevated temperature significantly affected the relative spore abundance of AMF species and decreases AMF diversity42, whereas elevated temperature has no significant effect on the composition of the AMF community in an alpine meadow ecosystem in China20 and a native grassland in the UK17. These inconsistent results may indicate different responses of AMF communities in different ecosystems to elevated temperature because the AMF species composition often is determined by environment and plant community composition23,44. In the current study, although elevated temperature significantly altered the development of AMF (Table 1), elevated temperature did not affect AMF spore population diversity and evenness; this result is consistent with the absence of a significant effect of elevated temperature on the species composition of the plant community41. However, an interactive effect of elevated temperature × N addition on AMF species richness was observed, which suggesting that the effects of elevated temperature on AMF species richness might be influenced by soil N availability. In the present study, elevated temperature decreased AMF spore density and diameter but increased the HLD significantly, which is consistent with a result from a simulated greenhouse experiment15, but not with the result from a field experiment in the Qinghai-Tibetan Plateau20. The distinction between our result and other results suggest that the influence of elevated temperature on AMF might be related to the climatic conditions of experiment ecosystem, such as, temperature. Our HLD results indicate that plants might have higher tolerance to high temperatures because an increase in HLD would increase the absorption of water and nutrients.
In the current study, N addition significantly decreased the species diversity and richness of the AMF spore population. These findings are consistent with earlier studies that show that N fertilization decreases the spore richness and abundance of AMF23,24,43. Moreover, N addition significantly reduced the biomass, spore density and diameter and HLD of AMF significantly, which is consistent with earlier results from Cedar Creek23,44, an alpine meadow ecosystem24. Based on the current results, the community composition and the development of AMF would respond quickly to N addition in the N-limited Songnen meadow ecosystem. In many studies, plant community composition determines the spore richness and biomass of AMF23,24,44. Therefore, the reduction of the richness and biomass of AMF in this ecosystem might be related to changes in the composition of plant community induced by N addition, given that our earlier study showed that N addition significantly increased the percentage in term of both number of plants and biomass of mycorrhizal species and reduced the percentage of non and poorly mycorrhizal species41.
In our study, significant interactive effects of elevated temperature × N addition on AMF spore density and diameter and species richness were observed. However, we did not detect significant interactive effects of elevated temperature × N addition on plant diversity, richness or aboveground productivity41. Thus, the factors that determine the species composition and development of AMF are likely different from those of plant communities.
A majority of the previous field and greenhouse studies demonstrate that AMF determine the structure and productivity of the plant community4,5,45,46. However, the species composition and the development of AMF are significantly influenced by global changes that include the following: N deposition23,24,44, warming41,42 and elevated CO215,23. Thus, changes in the species composition or in the development of AMF results in plant-soil feedbacks that regulate plant community structure and productivity36,47. However, the effects of plant-soil feedbacks on the plant community during climate change are not well understood. In the current study, although N addition significantly decreased the species richness and diversity of AMF spore population in the field experiment (Table 1), AMF significantly increased plant species diversity and richness via increasing the competition of non nitrophilous species (L. chinensis and S. viridis) with the addition of N in the greenhouse experiment (Fig. 1). These results are consistent with a previous study in which AMF reduced the negative effects of nitrogen enrichment on plant community structure in a dune grassland ecosystem48. The present results suggest that AMF might help improve the adaptability of plant species to N deposition and help maintain plant diversity in temperate meadow ecosystems due to AMF improved plant growth and increased plant biomass (Fig. 3). Furthermore, although elevated temperature had no impact on AMF species composition, AMF significantly increased plant species diversity and richness in the elevated temperature treatment condition (Fig. 1). Our results suggest that AMF improves growth of plants with high mycorrhizal benefits and tolerance to elevated temperatures, but further investigation is required to determine the mechanisms controlling the influence of AMF on the development of plant species under elevated temperature conditions.
AMF altered the relative abundance of plant species (%) in the elevated temperature and N addition treatments in the greenhouse experiment. The relative abundance of L. chinensis significantly increased in the elevated temperature, N addition and elevated temperature plus N addition treatments, but the relative abundance of S. corniculata decreased (Fig. 2) with AMF. This result might be related to the level of mycorrhizal dependence of different plant species; for example, AMF improved the seedling establishment of the mycorrhizal L. chinensis in the elevated temperature and N addition treatments but had no impact on the nonmycorrhizal plant S. corniculata. These results are consistent with the earlier findings that AMF increased the abundance and biomass of highly mycorrhizal species and reduced the abundance of nonmycorrhizal plants45,49. Moreover, the effects of AMF on plant community structure are affected by the species composition of the AMF community50,51,52. Therefore, under conditions of climate change, the feedbacks between the species of AMF and plant species should be considered in future studies. The species S. corniculata is a typical indication of grassland degradation caused by grazing and mowing in the Songnen meadow steppe and with the aggravation of degradation the number of S. corniculata highly increase53. Based on our results, AMF might reduce the threat of grassland degradation caused by climate change or overgrazing.
A large number of studies have demonstrated that AMF can improve aboveground productivity1,54,55. In the greenhouse experiment, AMF significantly increased total aboveground biomass which is consistent with the previous studies1,3,54. This result suggests that AMF still play a vital role in determining ecosystem net primary productivity under climate change of elevated temperature and N addition, which would affect carbon sequestration and influence C and N cycling in grassland ecosystem. In an earlier study, AMF were found to influence C and N cycling by altering the plant C:N:P stoichiometry56. However, the influence of AMF on plant C:N stoichiometry under elevated temperature and N addition conditions requires further study. The biomass of the plant community was also altered under the elevated temperature and N addition treatments by AMF. In both the elevated temperature and the N addition treatments, the proportion of biomass from L. chinensis and S. viridis increased significantly with AMF, whereas the proportion of biomass from S. corniculata decreased significantly. These results are consistent with early findings in a semiarid herbland in South Australia45. The changes in the composition of plant community biomass caused by AMF might be determined by the different mycorrhizal responses of plant species. In the current study, significant differences in mycorrhizal response among the species were observed (Fig. 3), positive mycorrhizal benefits of L. chinensis, S. viridis and L. davurica and negative mycorrhizal benefits of C. virgata and S. corniculata were detected. In the Songnen grassland, the species in the grass family (e.g., L. chinensis and S. viridis) are the most important forages, whereas the species in Chenopodiaceae family (e.g., S. corniculata) are weeds that livestock do not like to eat. Therefore, these results suggest that AMF can significantly improve the productivity of forage and reduce the productivity of harmful weeds, which would sustain livestock production. Furthermore, the plant species S. corniculata is a typical indicator of degradation in the Songnen grassland and our results indicate that that AMF can reduce the risk of grassland degradation under conditions of climate change by suppressing the growth of S. corniculata.
Conclusions
Elevated temperature and N addition treatments, proxies for global climate change, altered the spore population composition and suppressed the development of AMF. However, AMF reduced the negative effects of elevated temperature and N addition on plant diversity and productivity and increased the contribution of grasses thereby increasing the productivity of grassland. Thus, AMF have the potential to reduce the degradation of grasslands caused by elevated temperature and N addition.
Methods
Study site
The experiment was conducted at the Songnen Grassland Ecological Research Station (44°45′ N, 123°45′ E), Northeast Normal University, Jilin Province, in northeast China. The grassland is situated at the eastern edge of the Eurasian steppe and is characterized as Eurasian continental meadow steppe. Mean annual precipitation is approximately 400 mm, 90% of which occurs from May to October. Annual average air temperature is 4.9 °C and annual average land surface temperature is 6.2 °C. The soil in the study area is a soda-saline soil and the soil pH was 8.2, with 3–4% organic matter in the soil surface layer. The vegetation of the experimental site is dominated primarily by Leymus chinensis (Trin. Tzvel, Poaceae); Chloris virgata (Sw, Gramineae), Setaria viridis (L., Gramineae), Lespedeza davurica (Laxm., Leguminosae) and Suaeda corniculata (C. A. Mey, Chenopodiaceae) are the main companion species.
Field experiment: Effects of elevated temperature and N addition on the development and species composition of AMF
Experimental design
The experiment was a completely randomized block factorial experiment with two factors: elevated temperature and N addition. There were four treatments: control (C), elevated temperature (T), N addition (N) and elevated temperature plus N addition (T + N), with 6 replicates each. The size of the plots was 2 m × 3 m. The plots in the elevated temperature treatment were all heated continuously by infrared radiators (MSR-2420, Kalglo Electronics Inc., Bethlehem, PA, USA) that were suspended 2.25 m over the plot center. In each control or N addition plot, one ‘dummy’ heater of the identical shape and size was installed to mimic the shading effects of the infrared radiator. The heaters in the elevated temperature treatment were set for approximately 2000 W of radiation output. In northern, temperate grassland ecosystems, the saturation rate for N deposition is approximately 10 g m−2 yr−139. Therefore, in the N addition treatment; ammonium-nitrate (10 g m−2 yr−1) was added as an aqueous pulse on the first day of May each year. In the control and elevated temperature plots, an identical amount of water (without N) was added to account for that added in N addition condition. The experiment began in May 2006 and was terminated in September 2010.
Soil sampling
Three soil cores (15 cm in depth, 5 cm in diameter) were randomly collected and were mixed to create a composite sample at the end of the experiment. Fresh soil samples were sieved (2-mm) to remove roots and debris for AMF spore isolation.
Extraradical hyphae
The extraradical hyphae from the homogenized soils were extracted and stained with trypan blue using the method of Jakobsen et al.57. The hyphal length density (HLD) was quantified using a compound microscope with a gridded reticule at 250x magnification.
Spore separation and identification
The spores were extracted using wet sieving and sucrose centrifugation58, mounted on glass slides with alcohol lacto-glycerol (PVLG) and were examined under 100–1000x magnification with a microscope. The species of each spore was identified (Table S1) using taxonomic criteria59, the information published by INVAM (http://invam.caf.wvu.edu/) and Schüβler’s Glomeromycota Species List (http://www.lrz.de/~schuessler/amphylo/). The slides are maintained at Northeastern Normal University. A sporocarp was counted as a single spore. The spore density is the number of AM fungal spores in 20 g of soil. The average spore diameter was calculateed for each soil sample.
The species richness (SR) = number of AM fungal taxa found in 20 g of soil and the relative abundance (RA) = (number of spores of a species or genus/total spores) × 100. The number of spore population and the density were also used to calculate species richness (mean number of AMF species per 20 g of soil), diversity (Shannon-Weiner H′) and evenness.
Greenhouse experiment: The feedbacks of AMF on plant community composition and productivity
Soil and plants
The soil used in this experiment was collected from the grassland experimental sites. The soil was sterilized 2 times using high-pressure steam for 2 h at 121 °C per time. When the first sterilization completed, the soil were homogenized and then sterilized again. To isolate the AMF spores, we collected the soil (500 g) from the field plots under treatment for 5 years with elevated temperature and N addition. The isolated AMF spores were cultured using Medicago sativa with 200 g of sterilized soil. Plants were grown in phytotrons for 4 months with the temperature of 25 °C from 06:00–20:00, 18 °C from 20:00–06:00 and received deionized water to maintain soil moisture at 10–20% by weight. The entire cultured AMF from field experiment (including soil, root segments, AMF spores and hypha) was homogenized and used to inoculate the soils with AMF in the greenhouse experiment. The seeds of one dominant species L. chinensis and four companion species C. virgata, S. viridis, S. corniculata and L. davurica were collected from the Songnen meadow steppe and were stored in a refrigerator at <4 °C before use.
Experimental design
In the greenhouse experiment, the four treatments were identical to the treatments in field experiment, with control, elevated temperature, N addition and elevated temperature plus N addition. Each treatment was also replicated 6 times. Soil samples were collected from each treatment in the field experiment and were homogenized to use as AMF inocula in the greenhouse experiment. The sterilized soil was placed into pots and 200 g of AMF inoculum (approx. 6000 spores) from the similar treatment (from the field experiment) was added to the soils in the AMF treatment (AM). An identical amount of the inoculum mix was sterilized using 10 k Gy 60Co γ-rays and was used for the non-AMF (NM) treatment. To minimize differences in the rhizosphere microbial communities of AMF and non-AMF treatments, 10 ml filtrates free from mycorrhizal propagules from the inoculum were added to the non-AMF treatments and 10 ml deionized water were added to AMF treatments. The pots were 34 cm (in diameter) × 23 cm (in depth) and filled with 2.5 kg (dry weight) soil. The seeds of the five species were surface disinfected in 10% (v/v) hydrogen peroxide for 5 min, rinsed five times with deionized water and germinated at 20 °C. After 48 h, the germinated seeds were added to each pot and the density of these five species was thinned based on their densities in the field and was kept consistent for all treatments. The densities of these species were L. chinensis 15 plants per pot, S. viridis 10 plants per pot, C. virgata 7 plants per pot, S. corniculata 5 plants per pot and L. davurica 5 plants per pot.
The experimental pots were placed in phytotrons (LT/ACR-2002, E-Sheng Tech., Beijing, China) at Northeast Normal University. In the phytotrons, light intensity was 350 μ mol−2 S−1 (06:00–20:00) daily. The relative humidity was 40–60%. In the control and N addition treatments, the temperature settings were 22 °C from 06:00–10:00, 25 °C from 10:00–15:00, 22 °C from 15:00–20:00 and 22 °C from 20:00–06:00. In the elevated temperature and elevated temperature plus N addition treatments, the temperature in each time period was elevated 3 °C compared with the control and N addition treatments. The air temperature in the phytotrons was adjusted and monitored every 10 seconds and the temperature matched the mean summer temperature from 2000–2011 in the Songnen meadow grassland60. Ammonium nitrate (10 g m−2 yr−1) was added to the pots of N addition and elevated temperature plus N addition treatments. The pots were irrigated every 2 days; the soil water content was 50–60% of field capacity.
The plant species number and density of each species was observed and recorded daily at 14:00. The plants were harvested after twelve weeks of growth. The shoots and roots were removed from the pot and were washed with deionized water. The roots were cut into 1-cm segments and thoroughly mixed. A sub-sample of 0.5 g was cleared with 10% (w/v) KOH at 90 °C for 2 h and then stained with trypan blue. Mycorrhizal colonization (Table S2) was estimated according to a previously described method61. The shoots and remaining roots were dried at 60 °C for 48 h and then weighed to calculate plant productivity. The number and density of plant species were used to calculate the species diversity (Shannon-Weiner index H), richness and evenness. The mycorrhizal benefit was calculated according to the following formula. The mycorrhizal benefit = [(AM/NM) − 1] × 100.
Statistical analysis
The statistical analyses were conducted using the SPSS statistical software package 7 (SPSS 16.0 for Windows, Chicago, IL, USA). The spore density counts and estimates of species richness were square root transformed. Other data were analyzed without transformation. Spore density and AM fungal diversity index results are shown as arithmetic mean values with standard errors. A two-way ANOVA was used to test the effects of elevated temperature, N addition and their interaction with the AM fungal HLD, spore density, species richness, diversity and evenness in the field experiment and a three-way ANOVA was used to test the feedback of AMF on the community composition and productivity of the plant community in the greenhouse experiment. Treatment means were compared by Tukey at P = 0.05. The data were all tested for normality and homogeneity of variance before analysis.
Additional Information
How to cite this article: Zhang, T. et al. Response of AM fungi spore population to elevated temperature and nitrogen addition and their influence on the plant community composition and productivity. Sci. Rep. 6, 24749; doi: 10.1038/srep24749 (2016).
References
Schnitzer, S. A. et al. Soil microbes drive the classic plant diversity-productivity pattern. Ecology 92, 296–303 (2011).
Smith, S. E. & Smith, F. A. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu. Rev. Plant. Biol. 62, 227–250 (2011).
Wagg, C., Jansa, J., Schmid, B. & van Der Heijden, M. G. A. Belowground biodiversity effects of plant symbionts support aboveground productivity. Ecol. Lett. 14, 1001–1009 (2011).
van der Heijden, M. G. A. et al. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature, 396, 69–72 (1998).
Wagg, C., Bender, S. F., Widmer, F. & van Der Heijden, M. G. A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 111, 5266–5270 (2014).
Yang, G. et al. The interaction between arbuscular mycorrhizal fungi and soil phosphorus availability influences plant community productivity and ecosystem stability. J. Ecol. 102, 1072–1082 (2014).
Cheng, L. et al. Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2 . Science 337, 1084–1087 (2012).
Hodge, A., Campbell, C. D. & Fitter, A. H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413, 297–299 (2001).
Hodge, A. & Fitter, A. H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. USA 107, 13754–13759 (2010).
Govindarajulu, M. et al. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435, 819–823 (2005).
Jin, H. et al. The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol. 168, 687–696 (2005).
van der Heijden, M. G. A. Mycorrhizal fungi reduce nutrient loss from model grassland ecosystems. Ecology 91, 1163–1171 (2010).
Heinemeyer, A. & Fitter, A. H. Impact of temperature on the arbuscular mycorrhizal (AM) symbiosis: growth responses of the host plant and its AM fungal partner. J. Exp. Bot. 55, 525–534 (2004).
Rillig, M. C., Wright, S. F., Shaw, M. R. & Field, C. B. Artificial climate warming positively affects arbuscular mycorrhizae but decreases soil aggregate water stability in an annual grassland. Oikos 97, 52–58 (2002).
Staddon, P. L., Gregersen, R. & Jakobsen, I. The response of two Glomus mycorrhizal fungi and a fine endophyte to elevated atmospheric CO2, soil warming and drought. Global Change Biol. 10, 1909–1921 (2004).
Zavalloni, C. et al. Exposure to warming and CO2 enrichment promotes greater above-ground biomass, nitrogen, phosphorus and arbuscular mycorrhizal colonization in newly established grasslands. Plant Soil 359, 121–136 (2012).
Heinemeyer, A. et al. Impact of soil warming and shading on colonization and community structure of arbuscular mycorrhizal fungi in roots of a native grassland community. Global Change Biol. 10, 52–64 (2003).
Bell, C. W. et al. Linking microbial community structure and function to seasonal differences in soil moisture and temperature in a Chihuahuan desert grassland. Microbial Ecol. 58, 827–842 (2009).
Liu, Y. J. et al. Rapid change of AM fungal community in a rain-fed wheat field with short-term plastic film mulching practice. Mycorrhiza 22, 31–39 (2012).
Yang, W. et al. The arbuscular mycorrhizal fungal community response to warming and grazing differs between soil and roots on the Qinghai-Tibetan Plateau. Plos One, 8(9), e76447 (2013).
Clark, C. M. & Tilman, D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 451, 712–715 (2008).
Egerton-Warburton, L. M., Graham, R. C., Allen, E. B. & Allen, M. F. Reconstruction of the historical changes in mycorrhizal fungal communities under anthropogenic nitrogen deposition. P. Roy. Soc. Lond. B. Bio. 268, 2479–2484 (2001).
Antoninka, A., Reich, P. B. & Johnson, N. C. Seven years of carbon dioxide enrichment, nitrogen fertilization and plant diversity influence arbuscular mycorrhizal fungi in a grassland ecosystem. New Phytol. 192, 200–214 (2011).
Liu, Y. J. et al. Direct and indirect influences of 8 yr of nitrogen and phosphorus fertilization on Glomeromycota in an alpine meadow ecosystem. New Phytol. 194, 523–535 (2012).
Garcia, M. O., Ovasapyan, T., Greas, M. & Treseder, K. K. Mycorrhizal dynamics under elevated CO2 and nitrogen fertilization in a warm temperate forest. Plant Soil, 303, 301–310 (2008).
Ma, L. N. et al. The effects of warming and nitrogen addition on soil nitrogen cycling in a temperate grassland, Northeastern China. Plos One 6, e27645 (2011).
Li, Q. et al. Nitrogen addition and warming independently influence the belowground micro-food web in a temperate steppe. Plos One 8, e60441 (2013)
Shen, R. C., Xu, M., Chi, Y. G., Yu, S. & Wan, S. Q. Soil microbial responses to experimental warming and nitrogen addition in a temperate steppe of Northern China. Pedosphere 24, 427–436 (2014).
Kim, Y. C. et al. Arbuscular mycorrhizal fungal community response to warming and nitrogen addition in a semiarid steppe ecosystem. Mycorrhiza 25, 267–276 (2015).
Bever, J. D. Negative feedback within a mutualism: host-specific growth of mycorrhizal fungi reduces plant benefit. P. Roy. Soc. Lond. B. Bio. 269, 2595–2601 (2002).
Brinkman, E. P., Van Der Putten, W. H., Bakker, E. J. & Verhoeven, K. J. F. Plant-soil feedback: experimental approaches, statistical analyses and ecological interpretations. J. Ecol. 98, 1063–1073 (2010).
Casper, B. B. et al. Plant-soil feedback: Testing the generality with the same grasses in serpentine and prairie soils. Ecology 89, 2154–2164 (2008).
Bever, J. D., Westover, K. M. & Antonovics, J. Incorporating the soil community into plant population dynamics: the utility of the feedback approach. J. Ecol. 85, 561–573 (1997).
Bever, J. D. Soil community dynamics and the coexistence of competitors: Conceptual frameworks and empirical tests. New Phytol. 157, 465–473 (2003).
Mangan, S. A. et al. Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466, 752–756 (2010).
Bever, J. D., Platt, T. G. & Morton, E. R. Microbial population and community dynamics on plant roots and their feedbacks on plant Communities. Annu. Rev. Microbiol. 66, 265–283 (2012).
Zhang, Q. et al. Positive feedback between mycorrhizal fungi and plants influences plant invasion success and resistance to invasion. Plos One 5(8), e12380 (2010).
Zhu, T. C. Yang-cao Biological Ecology. (Jilin Science and Technology Press 2004).
Wang, Z. M., Song, K. S., Zhang, B. & Liu, D. W. Analyses of features of agro-climatic changes in Songnen plain in the past 40 years. Chinese Agr. Sci. Bull. 22, 241–246 (2006).
Bai, Y. et al. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from inner Mongolia Grasslands. Global Change Biol. 16, 358–372 (2010).
Zhang, T., Guo, R., Gao, S., Guo, J. X. & Sun, W. Responses of plant community composition and biomass production to warming and nitrogen deposition in a temperate meadow ecosystem. Plos One 10(4), e0123160 (2015).
Sun, X. et al. Diversity of arbuscular mycorrhizal fungal spore communities and its relations to plants under increased temperature and precipitation in a natural grassland. Chinese Sci. Bull. 58, 4109–4119 (2013).
Egerton-Warburton, L. M., Johnson, N. C. & Allen, E. B. Mycorrhizal community dynamics following nitrogen fertilization: A cross-site test in five grasslands. Ecol. Monogr. 77, 527–544 (2007).
Johnson, N. C., Rowland, D. L., Corkidi, L., Egerton-Warburton, L. M. & Allen, E. B. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84, 1895–1908 (2003).
O’connor, P. J., Smith, S. E. & Smith, E. A. Arbuscular mycorrhizas influence plant diversity and community structure in a semiarid herbland. New Phytol. 154, 209–218 (2002).
van der Heijden, M. G. A. & Horton, T. R. Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J. Ecol. 97, 1139–1150 (2009).
Casper, B. B. & Castelli, J. P. Evaluating plant-soil feedback together with competition in a serpentine grassland. Ecol. Lett. 10, 394–400 (2007).
van der Heijden, M. G. A., Verkade, S. & De Bruin, S. J. Mycorrhizal fungi reduce the negative effects of nitrogen enrichment on plant community structure in dune grassland. Global Change Biol. 14, 2626–2635 (2008).
Wolfe, B. E., Weishampel, P. A. & Klironomos, J. N. Arbuscular mycorrhizal fungi and water table affect wetland plant community composition. J. Ecol. 94, 905–914 (2006).
Hartnett, D. C. & Wilson, G. W. T. Mycorrhizae influence plant community structure and diversity in tallgrass prairie. Ecology 80, 1187–1195 (1999).
van der Heijden, M. G. A. et al. The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol. 172, 739–752 (2006).
Puschel, D., Rydlova, J. & Vosatka, M. Mycorrhiza influences plant community structure in succession on spoil banks. Basic. Appl. Ecol. 8, 510–520 (2007).
Zhou, D., Li, Q., Song, Y. & Wang, X. Salinization-alkalization of Leymus chinensis grassland in Songnen Plain of northeast China. -Chin. J. Appl. Ecol. 22, 1423–1430 (2011)
Bauer, J. T., Kleczewski, N. M., Bever, J. D., Clay, K. & Reynolds, H. L. Nitrogen-fixing bacteria, arbuscular mycorrhizal fungi and the productivity and structure of prairie grassland communities. Oecologia 170, 1089–1098 (2012).
Mccain, K. N. S., Wilson, G. W. T. & Blair, J. M. Mycorrhizal suppression alters plant productivity and forb establishment in a grass-dominated prairie restoration. Plant Ecol. 212, 1675–1685 (2011).
Johnson, N. C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 185, 631–647 (2010).
Jakobsen, I., Abbott, L. K. & Robson, A. D. External hyphae of vesicular arbuscular mycorrhizal fungi associated with Trifolium subterraneum L.2. hyphal transport of 32P over defined distances. New Phytol. 120, 509–516 (1992).
McKenney, M. C. & Lindsey, D. L. Improved method for quantifying endomycorrhizal fungi spores from soil. Mycologia 79, 779–782 (1987).
Schenck, N. C. & Perez, Y. Manual for the identification of VA mycorrhizal fungi. Gainesville, FL., USA: Synergistic Publications, (1990).
Li, Z. et al. The influence of precipitation regimes and elevated CO2 on photosynthesis and biomass accumulation and partitioning in seedlings of the rhizomatous perennial grass Leymus chinensis. Plos One 9(8), e103633 (2014).
Trouvelot, A., Fardeau, J. C., Plenchette, C., Gianinazzi, S. & Gianinazzapearson, V. Nutritional balance and symbiotic expression in mycorrhizal wheat. Physiol Veg 24, 300–300 (1986).
Acknowledgements
This work was funded by the National Natural Science Foundation of China (31300097 and 31470405), State Key Laboratory of Desert and Oasis Ecology, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences. And Jilin Provincial Education Department (2013–5) are also appreciated.
Author information
Authors and Affiliations
Contributions
T.Z., X.Y. and J.X.G. designed the research; T.Z. and X.Y. performed the research; T.Z., X.Y. and R.G. analyzed the data; and T.Z., X.Y., R.G. and J.X.G. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, T., Yang, X., Guo, R. et al. Response of AM fungi spore population to elevated temperature and nitrogen addition and their influence on the plant community composition and productivity. Sci Rep 6, 24749 (2016). https://doi.org/10.1038/srep24749
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep24749
This article is cited by
-
Sensitivity of soil fungal and bacterial community compositions to nitrogen and phosphorus additions in a temperate meadow
Plant and Soil (2022)
-
Septoglomus species dominate the arbuscular mycorrhiza of five crop plants in an arid region of northern Mexico
Symbiosis (2022)
-
Arbuscular mycorrhizae: natural modulators of plant–nutrient relation and growth in stressful environments
Archives of Microbiology (2022)
-
Arbuscular mycorrhizal fungi associated with Phoenix dactylifera L. grown in Tunisian Sahara oases of different salinity levels
Symbiosis (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.