Abstract
Hierarchical TiO2 micron spheres assembled by nano-plates were prepared through a facile hydrothermal route. Chemical tuning of the TiO2 through hydrogen reduction (H-TiO2) is shown to increase oxygen-vacancy density and thereby modifies the electronic properties. H-TiO2 spheres with a polar surface serve as the surface-bound intermediates for strong polysulfides binding. Under the restricting and recapturing effect, the sulfur cathode could deliver a high reversible capacity of 928.1 mA h g−1 after 50 charge-discharge cycles at a current density of 200 mA g−1. The H-TiO2 additive developed here is practical for restricting and recapturing the polysulfide from the electrolyte.
Similar content being viewed by others
Introduction
Advanced power sources are urgently needed to meet the continuously surging demand in electric vehicles and large-scale electrochemical energy-storage systems1,2,3,4,5. However, current Li-ion batteries based on intercalation compound (e.g., LiCoO2 and LiFePO4) cannot satisfy this demand in terms of specific energy6,7,8. Li-S batteries, with a remarkably high theoretical energy density of 2567 Wh kg−1 and a high specific capacity of 1672 mA h−1 g−1, have recently attracted intense interest1,9,10,11. Because of the low cost, non-toxicity and natural abundance, Li-S batteries show great promise for large-scale applications in renewable energy fields2,12. However, Li-S cells generally suffer from low sulfur utilization and poor long-term cycling stability mainly because of the dissolution of polysulfide in liquid electrolytes, which triggers a “shuttle effect” process6,9,13,14.
To solve these problems, major efforts have been centered on the development of the constraint of polysulfide dissolution12,14,15,16,17,18. More approaches, to date, have been devoted to physically confining the polysulfide within porous carbon materials such as mesoporous carbon19,20,21, graphene22,23,24 and hollow carbon spheres20,25. However, the polysulfide tends to diffuse out from the hydrophobic pores of carbon materials. This may be because carbon, being non-polar in nature, does not bind favourably with the polar and ionic sulfides8. Cui Yi group shown that sulfur–TiO2 yolk–shell composite could achieve a high specific capacity26. Next year, they further demonstrated that the strong chemical bonds between TinO2n−1 and S-species made important contribution to the improvement of the electrochemical properties27. Therefor, one of the targeted approaches is to bind polysulfides onto hydrophilic metal oxide.
Here, we report a strategy to effectively entrap polysulfides by a TiO2 host nanosheets with a polar surface. The hierarchical TiO2 spheres consisted of nano-plates were synthesised through a hydrothermal route. TiO2 spheres with a polar surface serve as the surface-bound intermediates for strong polysulfides binding (Fig. 1)8,12. After 50 cycles, it still has a capacity of 928.1 mA h g−1, corresponding to a capacity retention of 71%. The fine performances of S + H-TiO2 electrodes also demonstrated the critical role of H-TiO2 additive in mitigating the polysulfide dissolution into the electrolyte.
Results and Discussion
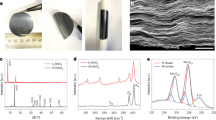
Figure 2a shows a typical scanning electron microscopy (SEM) image of the TiO2. Interestingly, the hierarchical TiO2 micron spheres are composed of TiO2 nano-plates. The nano-plates are arranged in spatial divergence form, which can be directly elucidated from the collapsed spheres (Fig. 2b). Highly ordered nano-plates are located in the core and arranged along the radial orientation to the outer surface. Hollow interior structure of as-obtained TiO2 spheres are also observed by TEM, as shown in Fig. 2c. Therefore, the hierarchically porous and hollow structure provide high surface area to adsorb the polysulfide dispersed in electrolyte. The TEM image of nano-plates in Fig. 1d further reveals that the component unit was fan-shaped plates with the diameter of about 70 nm and the radius of 500 nm. Fig. 2e shows the HRTEM image of an individual TiO2 plate with a lattice spacing of 0.35 nm, which is in good agreement with the d-spacing of (110) planes as observed in the inset of its corresponding Fast Fourier Transform pattern.
The electrical conductivity of TiO2 can be substantially enhanced, after annealing in a reducing gas atmosphere9,28. The anatase TiO2 and hydrogen reduced TiO2 were characterized by XRD (Fig. 3a). The identified peaks are indexed as anatase TiO2 (JCPDS card No. 84–1285) and no observable structural differences are observed between TiO2 and H-TiO2. There were also no obvious morphological and structure changes observed for H-TiO2 upon hydrogen treatment (Figure S1). To further probe the electronic and chemical environment, X-ray photoelectron spectroscopy (XPS) analysis was performed. Both spectras were calibrated with respect to the C 1s peak at 284.5 eV for clear comparison. Figure 3b (insets) illustrates the Ti 2p core level spectra for anatase TiO2 and H-TiO2. The anatase TiO2 sample shows two predominant peaks at 464.3 and 458.6 eV, which are attributed to the characteristic Ti 2p1/2 and Ti 2p3/2 peaks of Ti4+. In comparison to anatase TiO2, the peaks of the H−TiO2 sample show a red shift in binding energy, suggesting that they have different bonding environments. By subtracting the normalized Ti 2p spectra of H−TiO2 with TiO2 sample, there are two extra peaks centered at ca. 463.4 and 458.1 eV (Fig. 3b). These two peaks are within the range of published Ti3+ 2p binding energies9,28,29,30. The result suggests that oxygen vacancies (Ti3+ sites) are created in H−TiO2 sample during hydrogenation. It has been reported that the less positively charged Ti3+ nuclei leads to an increment in electron density and therefore a weaker binding effect9.
Restricting and recapturing of polysulfide diffusion from additive H-TiO2 were clearly demonstrated through an in-situ visual–electrochemical study. The designed S and S + H-TiO2 cathodes are cycled in sealed vials against Li anodes with the same electrolyte as used in coin cells. As shown in the photograph in Fig. 4a–c, the electrolyte with S cathode changed from colourless to bright yellow during 250 cycles under the scan rate of 1 mv s−1. The colour change indicated that the polysulfides diffuse out of the cathode and dissolves in the electrolyte. However, the electrolyte with S + H-TiO2 cathode showed no obvious change, demonstrating the restriction of polysulfide diffusion from the H-TiO2 component. After 250 CV cycles, H-TiO2 was added to the bottle with pure S cathode and shook for several seconds. After resting for 2 hours, the turbid electrolyte turned clear as H-TiO2 precipitated and its yellow color faded simultaneously. This demonstrates that the H-TiO2 additive in these cathodes also shown efficient adsorption properties for the escaped polysulfide.
To further demonstrate the interaction of polysulfide and H-TiO2, we studied the precipitate from the sealed vial (shown as Fig. 4e). On the basis of the energy-dispersive X-ray spectroscopy (EDS) mappings (Fig. 5a–d), we conclude that element sulfur is homogeneously absorbed on the surface of H-TiO2. In the XPS S 2p spectra (Fig. 5e), various sulfur bonds can be observed, which also confirmed the existence of S. The peak at 166–170 eV is related to the electrolyte. As reported in previous studies, the peak in the range of 162–163 eV can be assigned to Li2Sx species9. Therefore, we confirmed that the H-TiO2 spheres were efficient in adsorption of polysulfide and deposition of S species. The S + H-TiO2 cathode after 25 cycles (Fig. 5f) shows little precipitation of insoluble products on the surface of the H-TiO2 particles.
(a–d) SEM image, EDX elemental mapping of Ti, O, S and (e) S 2p XPS spectra of the precipitation gathered from the sealed vial in Fig. 3e. (e) SEM images of S + H−TiO2 cathode after the 25th charge.
Figure 6 depicts the typical cyclic voltammetry (CV) curves at a constant scan rate of 0.1 mV/s in the voltage range of 1.5–3.0 V. During the first cycle, two pronounced cathodic peaks at approximately 2.29 and 1.98 V are observed. According to the previously reported mechanism, the peak at 2.29 V corresponds to the transition from elemental sulfur to long-chain lithium polysulfides (Li2Sn, 4 < n < 8)6,9. The peak at 1.98 V is related to the further reduction of low-order lithium polysulfides to Li2S2 and Li2S. The oxidation peak at about 2.52 V is associated with the reverse reactions in the charging stage. In the second CV scan, the cathodic peak at 1.98 V of the initial cathodic peak shifts to the position at about 2.01 V and the current of the corresponding peak decreases to a low value. In the subsequent anodic cycle (3–6th cycles), the oxidation peaks shift to the position at 2.49 V, which are believed to be attributed to the complete conversion of Li2S into elemental S the formation of Li2Sn (n > 2)11,31. Peaks located at 1.72 V and 2.03 V reveal the lithiation and delithiation of anatase TiO2.
To further study the electrochemical properties, galvanostatic cycling was performed at a current density of 200 mA g−1. The S + TiO2 and S + H-TiO2 electrodes delivered an initial discharge capacities of 1339.4 and 1301.9 mA h g−1, respectively. Among them, the S + H-TiO2 electrode exhibited the best capacity retention, as shown in Fig. 7a. After 50 cycles, it still has a capacity of 928.1 mA h g−1, corresponding to a capacity retention of 71%. After 100 cycles at a current density of 1 A g−1, it still has a capacity of 407 mA h g−1 (Fig. 2s). The S + TiO2 electrode showed relatively poor cycling stability, but much better than that of the pure sulfur electrode. The best performance of S + H-TiO2 electrode also demonstrated that the critical role of H-TiO2 additive in mitigating the polysulfide dissolution into the electrolyte.
Figure 7b displays the charge–discharge curves of the S + H-TiO2 electrode cathode. Two well-defined discharge plateaus were observed, which could be assigned to the two-step reaction of sulfur with lithium. The first plateau, centered around 2.35 V, was generally attributed to the reduction of the S8 ring and the formation of S82−. The discharge plateau at 2.08 V was ascribed to the further reduction of the higher polysulfides (Li2Sn, 4 ≤ n ≤ 8) to the lower polysulfides (Li2Sn, n ≤ 3). There exhibited two plateaus in the charge process at about 2.37 and 2.47 V, respectively. Peaks at the beginning charging profile can be attributed to H-TiO2. The positions of the plateaus were in accordance with the typical peaks of the S + H-TiO2 electrode in the CV profiles. Within the first 20 cycles, the plateaus were well preserved. In longer cycling, the capacity only dropped from 1081.3 to 928.1 mA h g−1, indicating that the H-TiO2 additive was effective in preventing the long-term loss of sulfur into the electrolyte during the redox processes. These behaviors of S + H-TiO2 electrode in the coin cells were consistent with what was observed in the sealed vials, where the H-TiO2 additive showed effective polysulfide restricting and recapturing ability.
The S + H-TiO2 cathodes also show excellent cycling stability under continuously varying current densities, as shown in Fig. 8a. A reversible high and stable capacity of 850.8 mA h g−1 was retained after 5 cycles at a current rate of 0.4 A g−1. At a high current rate of 0.6 A g−1, the S + H-TiO2 electrode can give a reversible capacity of 650 mAh/g. When the current was further increased to 1.2 A g−1, a high capacity of 466.4 mAh/g can still be achieved, indicating fast reaction kinetics. It is worth mentioning that when the current was abruptly switched from 1.2 A g−1 to 0.4 A g−1 again, the capacity were recovered to 650.6 mA h g−1, indicating the superior robustness and reversibility of S + H-TiO2 electrode. The electrochemical impedance spectroscopy (EIS) was also performed to study internal resistance of the S + H-TiO2 and the corresponding Nyquist plots are shown in Fig. 8b. All the impedance plots for charged to 3.0 V are composed of a semicircle in the high-frequency region relating to the charge transfer resistance of the cathode and a sloping, straight line in the low-frequency region corresponding to the Li-ion diffusion within the cathode. The intersection of the semicircle on the real axis provides an approximate indication of the charge transfer resistance (Rct). This indicates that the slow accumulation of Li2S on the surface of S + H-TiO2 cathode slightly caused the increase of Rct and the capacity degradation with cycle processing.
Conclusions
Hierarchical TiO2 spheres consisting of nano-plates reduced through hydrogen provide polysulfides host material as a cathode additive. The reduced TiO2 spheres with a polar surface serve as the surface-bound intermediates for strong polysulfides binding. After 50 cycles, it still has a capacity of 928.1 mA h g−1, corresponding to the capacity retention of 71%. The fine performances from S + H-TiO2 electrodes also confirm that the critical role of H-TiO2 additive in restricting and recapturing the polysulfide from the electrolyte.
Methods
Preparation of Cathode Composites
In a typical synthesis, 1 mL of titanium tetrachloride and hydrofluoric acid were added to 40 mL of ethyl alcohol. After stirring for a few minutes, the solution was then transferred to a 50 mL Teflon-lined stainless steel autoclave and kept in an electric oven at 200 °C for 6 h. The autoclave was then taken out of the oven and left to cool naturally to room temperature. The white precipitate was harvested via centrifugation, washed thoroughly with ethanol and dried at 60 °C overnight. Hydrogen treatment of TiO2 was performed by a thermal annealing process in an Ar (with H2 10%) gas flow. The temperature was increased from room temperature at a rate of 2 °C /min and held at 500 °C for 3 h. The TiO2 or H-TiO2 additive (80 mg) and sulfur (160 mg) were ground together for 15 min.
Materials Characterization
The crystalline phase of TiO2 and H-TiO2 was identified by X-ray diffraction (XRD, Dmax/2500PC, Rigaku, Japan) with Cu Kα radiation (λ = 1.5406 Å). Morphology and structure of the samples were characterized by a transmission electron microscope (TEM, JEM-2100F, 200 kV) and a field emission scanning electron microscope (SEM, JSM-6700F/Japan or FEIXL-30/USA with genesis 2000 energy-dispersive X-ray spectroscopy attachment). X-ray photo-electron spectrometry with an ESCALAB250 analyzer (XPS) were employed to characterize the obtained samples. Thermal gravimetric analysis (SDT Q600, TA Instruments Inc. USA) was carried out to estimate the amount of sulfur under Ar flow (100 mL min−1) at a heating rate of 10 °C min−1.
Electrochemical Measurements
The TiO2-additive and sulfur mixtures were mixed with Super-P carbon black and PVDF binder, with a mass ratio of 60:20:20, in N-methyl-2-pyrrolidone to produce electrode slurries. The resulting slurries were then casted on carbon fiber paper (15 g m–2, Jiangsu China) with a laboratory doctor blade. The prepared electrode sheets were dried at 60 °C for 12 h in a vacuum oven. The typical areal density loading of active S was 0.8-1.3 mg/cm2. CR2025-type half-coin cells were assembled in an argon-filled glove box with the H2O and O2 contents below 1 ppm. Metallic lithium foil was used as the counter and reference electrode. Lithium bis-(trifluoromethanesulfonyl)imide (LiTFSI, 1 M) in cosolvent of 1,3-dioxolane and 1,2-dimethoxyethane (volume ratio 1:1) with lithium nitrite (LiNO3, 0.1 M) was used as electrolyte. Charge-discharge performances were evaluated by a LAND CT2001A battery instrument at a constant current density within a voltage window of 1.5–3.0 V at room temperature. Cyclic voltammogram measurements were carried out on an electrochemical workstation (CHI650D, Shanghai Chen Hua Instruments Ltd) at a scan rate of 0.1 mV s−1 from 1.5 to 3.0 V.
Additional Information
How to cite this article: Yang, Z.-Z. et al. Hierarchical TiO2 spheres as highly efficient polysulfide host for lithium-sulfur batteries. Sci. Rep. 6, 22990; doi: 10.1038/srep22990 (2016).
References
Xu, C. H. et al. Graphene-based electrodes for electrochemical energy storage. Energy Environ. Sci. 6, 1388–1414 (2013).
Yin, Y. X., Xin, S., Guo, Y. G. & Wan, L. J. Lithium-sulfur batteries: electrochemistry, materials and prospects. Angew. Chem. Int. Ed. Engl. 52, 13186–13200 (2013).
Zhang, X. L. et al. Improved cycle stability and high security of Li-B alloy anode for lithium-sulfur battery. J. Mater. Chem. A 2, 11660–11665 (2014).
Si, W. et al. A single rolled-up Si tube battery for the study of electrochemical kinetics, electrical conductivity and structural integrity. Adv. Mater. 26, 7973–7978 (2014).
Zhou, Y. et al. Effect of carbon coating on low temperature electrochemical performance of LiFePO4/C by using polystyrene sphere as carbon source. Electrochim. Acta 56, 5054–5059 (2011).
Lin, Z. & Liang, C. Lithium–sulfur batteries: from liquid to solid cells. J. Mater. Chem. A 3, 936–958 (2015).
Manthiram, A., Fu, Y. & Su, Y. S. Challenges and prospects of lithium-sulfur batteries. Acc. Chem. Res. 46, 1125–1134 (2013).
Pang, Q., Kundu, D., Cuisinier, M. & Nazar, L. F. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat Commun 5, 4759 (2014).
Liang, Z. et al. Sulfur cathodes with hydrogen reduced titanium dioxide inverse opal structure. ACS Nano 8, 5249–5256 (2014).
Cui, X. L., Shan, Z. Q., Cui, L. & Tian, J. H. Enhanced electrochemical performance of sulfur/carbon nanocomposite material prepared via chemical deposition with a vacuum soaking step. Electrochim. Acta 105, 23–30 (2013).
Zhang, Z. W. et al. 3D Interconnected Porous Carbon Aerogels as Sulfur Immobilizers for Sulfur Impregnation for Lithium-Sulfur Batteries with High Rate Capability and Cycling Stability. Adv. Funct. Mater. 24, 2500–2509 (2014).
Wang, Z. et al. Enhancing lithium-sulphur battery performance by strongly binding the discharge products on amino-functionalized reduced graphene oxide. Nat Commun 5, 5002 (2014).
Cui, Y., Abouimrane, A., Sun, C. J., Ren, Y. & Amine, K. Li-Se battery: absence of lithium polyselenides in carbonate based electrolyte. Chem. Commun. 50, 5576–5579 (2014).
Kim, J. H. et al. Hydroxylated carbon nanotube enhanced sulfur cathodes for improved electrochemical performance of lithium-sulfur batteries. Chem. Commun. 51, 13682–13685 (2015).
Zhang, C., Wu, H. B., Yuan, C., Guo, Z. & Lou, X. W. Confining sulfur in double-shelled hollow carbon spheres for lithium-sulfur batteries. Angew. Chem. Int. Ed. Engl. 51, 9592–9595 (2012).
Zhou, W., Yu, Y., Chen, H., DiSalvo, F. J. & Abruna, H. D. Yolk-shell structure of polyaniline-coated sulfur for lithium-sulfur batteries. J. Am. Chem. Soc. 135, 16736–16743 (2013).
Wang, H. et al. Graphene-wrapped sulfur particles as a rechargeable lithium-sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 11, 2644–2647 (2011).
Yang, Y., Zheng, G. & Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 42, 3018–3032 (2013).
Zhou, X. Y. et al. Improving the performance of lithium-sulfur batteries by graphene coating. J. Power Sources 243, 993–1000 (2013).
Sun, F. et al. High efficiency immobilization of sulfur on nitrogen-enriched mesoporous carbons for Li-S batteries. ACS Appl Mater Interfaces 5, 5630–5638 (2013).
Yao, Y. L., Liu, H. C., Li, G. C., Peng, H. R. & Chen, K. Z. Synthesis and electrochemical performance of phosphate-coated porous LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion batteries. Electrochim. Acta 113, 340–345 (2013).
Rong, J., Ge, M., Fang, X. & Zhou, C. Solution ionic strength engineering as a generic strategy to coat graphene oxide (GO) on various functional particles and its application in high-performance lithium-sulfur (Li-S) batteries. Nano Lett. 14, 473–479 (2014).
Lu, S., Chen, Y., Wu, X., Wang, Z. & Li, Y. Three-dimensional sulfur/graphene multifunctional hybrid sponges for lithium-sulfur batteries with large areal mass loading. Sci. Rep. 4, 4629 (2014).
Ji, X., Evers, S., Black, R. & Nazar, L. F. Stabilizing lithium-sulphur cathodes using polysulphide reservoirs. Nat Commun 2, 325 (2011).
Zhou, W., Xiao, X., Cai, M. & Yang, L. Polydopamine-coated, nitrogen-doped, hollow carbon-sulfur double-layered coreαshell structure for improving lithium-sulfur batteries. Nano Lett. 14, 5250–5256 (2014).
Wei Seh, Z. et al. Sulphur-TiO2 yolk-shell nanoarchitecture with internal void space for long-cycle lithium-sulphur batteries. Nat Commun 4, 1331 (2013).
Tao, X. et al. Strong sulfur binding with conducting Magneli-phase Ti(n)O2(n-1) nanomaterials for improving lithium-sulfur batteries. Nano Lett. 14, 5288–5294 (2014).
Lu, X. et al. Hydrogenated TiO2 nanotube arrays for supercapacitors. Nano Lett. 12, 1690–1696 (2012).
Bliokh, K. Y., Dressel, J. & Nori, F. Conservation of the spin and orbital angular momenta in electromagnetism. New J. Phys. 16, 093037 (2014).
Mayer, J. T., Diebold, U., Madey, T. E. & Garfunkel, E. Titanium and reduced titania overlayers on titanium dioxide(110). J. Electron. Spectrosc. Relat. Phenom. 73, 1–11 (1995).
Jayaprakash, N., Shen, J., Moganty, S. S., Corona, A. & Archer, L. A. Porous Hollow Carbon@Sulfur Composites for High-Power Lithium-Sulfur Batteries. Angew. Chem. 123, 6026–6030 (2011).
Acknowledgements
The authors acknowledge financial support from the Fundamental Research Funds for Jilin University (JCKY-QKJC02), the Science and Technology Development Project of Jilin Province, the ChangBai Mountain Scholars Program (2013014) and Project 985-Materials Science and Engineering of Jilin University.
Author information
Authors and Affiliations
Contributions
Z.Y. and H.W. conceived the work. Z.Y., L.L., C.W. and X.Z. performed the experiments. Z.Y., H.W., J.W. and Q.J. analyzed the data and prepared the manuscript. All authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, ZZ., Wang, HY., Lu, L. et al. Hierarchical TiO2 spheres as highly efficient polysulfide host for lithium-sulfur batteries. Sci Rep 6, 22990 (2016). https://doi.org/10.1038/srep22990
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep22990
This article is cited by
-
Effects of different GO contents in GO@KB-S composite prepared by spray drying method for lithium-sulfur batteries
Ionics (2020)
-
Mesoporous TiO2 Nanofiber as Highly Efficient Sulfur Host for Advanced Lithium–Sulfur Batteries
Chinese Journal of Mechanical Engineering (2019)
-
TiO2/Porous Carbon Composite-Decorated Separators for Lithium/Sulfur Battery
Nanoscale Research Letters (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.