Abstract
It has been universally delineated that the plasmonic metal nanoparticles can enhance the efficiency of photovoltaic cell by increasing the probability of energetic solar photons capturing phenomena using localized surface plasmonic resonance response. In this paper, we developed a novel in-situ simple approach to synthesize noble plasmonic silver nanoparticles (AgNP) from aqueous poly-vinyl-pyrrolidone solution of metal salt using radiolysis of water via synchrotron monochromatic X-ray irradiation without any chemical reducing agent. X-ray irradiation of water produces hydrated electrons  , superoxide
, superoxide  and atom radicals
and atom radicals  , which triggers the reaction and reduces metal salt. X-ray radiolysis based synthesis provides the control over the reaction and prevent the formation of secondary products as occurs in case of chemical reduction route. In the previous studies, synchrotron “white” X-rays had been examined for the synthesis of metal nanoparticles, but that technique limits only upto the material synthesis while in this work we explored the role of “monochromatic” X-rays for the production of bulk amount of nanoparticles which would also provide the feasibility of in-situ characterization. Transmission electron micrographs show that the synthesized AgNP appears spherical with diameter of 2–6 nm and is in agreement with the size estimation from uv-vis spectra by “Mie theory”.
, which triggers the reaction and reduces metal salt. X-ray radiolysis based synthesis provides the control over the reaction and prevent the formation of secondary products as occurs in case of chemical reduction route. In the previous studies, synchrotron “white” X-rays had been examined for the synthesis of metal nanoparticles, but that technique limits only upto the material synthesis while in this work we explored the role of “monochromatic” X-rays for the production of bulk amount of nanoparticles which would also provide the feasibility of in-situ characterization. Transmission electron micrographs show that the synthesized AgNP appears spherical with diameter of 2–6 nm and is in agreement with the size estimation from uv-vis spectra by “Mie theory”.
Similar content being viewed by others
Introduction
Plasmonic metal nanoparticles are of great interest in the field of green energy especially solar cell industry1. They build the foundation for enhanced light trapping in the device via their unique localized surface plasmon resonance response (LSPR) which could be easily tuned by optimising the size and shape of the nanoparticles2,3,4. For the application of solar cell, material required should be of high purity grade without any secondary product contamination. Numerous research activities have been reported to attempt these nanoparticles by chemical route in liquid5, gas phase6 and under high vacuum environment7. But most of the methods limit to control the reaction rate and the formation of secondary products. Radiation induced radiolysis synthesis overcome this problem and provides simple physico-chemical reaction, control over the reaction rate without any contamination under room temperature and atmospheric pressure8,9. Proton10, electron11, gamma12 and X-ray beams13 are the suitable irradiants to induce the reaction by the action of radiolysis of water, results in the generation of hydrated electrons  which plays the role of strong reducing agent towards metal ions14. Several attempts has been imparted to synthesize the metal nanoparticles by these radiation induced techniques, out of which gamma rays provide the low polydispersity product but lags behind due to their constrain over the safety concerns, irradiation time and in-situ characterization. On the other hand X-rays resolve these issues, provides longer irradiation time (as long as required), in-situ characterization13 and are widely available at laboratory and synchrotron light source.
which plays the role of strong reducing agent towards metal ions14. Several attempts has been imparted to synthesize the metal nanoparticles by these radiation induced techniques, out of which gamma rays provide the low polydispersity product but lags behind due to their constrain over the safety concerns, irradiation time and in-situ characterization. On the other hand X-rays resolve these issues, provides longer irradiation time (as long as required), in-situ characterization13 and are widely available at laboratory and synchrotron light source.
Synchrotron light source provides the X-rays of variable energy with fine control over the wavelength which could be optimized for the desired application. X-ray energy is efficient to trigger the reaction by dosimeteric based radiolysis process, as it commands the reaction rate mechanism and provides the facility to investigate the element specific electronic structural properties15. Many research groups are indulged to use the synchrotron X-rays for the formation of metallic nanoparticles. Neal N. Cheng16 reported the chemical enhancement by NPs under the irradiation of X-rays. X-ray enhance the reaction yield resulting in the increased chemical properties. Yung-Chin Yang17 and Kuan-Nan Lin18 performed the synthesis of gold nanoparticles by controlling their size and shape using irradiation of synchrotron “white” X-ray beam. Qing Ma19 successfully achieved the formation of metallic pattern/structure on the substrate which would lead to next generation of electronics. One pot synthesis protocol for metallic (AuPt, AuNi) nanocomposites has been attained using synchrotron radiation by Cheng-Liang Wang20 and Chong-Cook Kim21. All such experiments were performed under synchrotron “white” X-ray beam, while S. Remita reported the synthesis of Ag-nanoparticles with highly focused monochromatic X-ray along with in-situ UV-vis spectroscopy as well as SAXS measurements13. We achieved the desired task with monochromatic, unfocused and low integrated flux density X-ray beam that allows the formation of metallic nanoparticle in bulk quantity. Since the experiment is successfully conducted at much lower flux as compared to previous monochromatic focused beam experiments by S. Remita13, this opens the possibility of synthesis of plasmonic nanoparticles with high power laboratory based X-ray sources (LXS) thereby making it feasible to wide range of users from research and industry. Although “white” X-rays take lesser time in the process of synthesizing the nanoparticles than “monochromatic” X-rays, but still it dominate the white-beam because of its unique feasibility to provide the in-situ material characterization. X-ray techniques based in-situ characterization provides the possibility of investigating average particle size using SAXS13, electronic structure and chemical bonding using NEXAFS/EXAFS22,23, topology/morphology using nano-resolution imaging24 and crystalline structure using X-ray diffraction. This feature of monochromatic beam makes our method novel for the production of bulk amount of plasmonic nanoparticles along-with online optical & structural characterization and opens the gateway of new era for plasmonic research. This will also be helpful in controlling and tuning the properties of synthesized plasmonic nanoparticles through optimized irradiation dose and X-ray energy. Therefore synchrotron radiation based synthesis, though being expensive, is the only way to investigate the real-time electronic structure evolution with the growing size of nanoparticles, which is still a mystery. In this research work, we have developed a novel in-situ synthesis protocol for plasmonic silver nanoparticles (AgNP) from aqueous poly-vinyl-pyrrolidone (PVP) solution of metal-salt using synchrotron monochromatic x-ray without any chemical reducing agent, to make a better control over size and shape by optimizing the energy and exposure time. The effect of monochromatic beam energy at constant exposed radiation dose on structural properties of nano-particles formed is also studied.
Methods
Silver nitrate and PVP of high purity grade were purchased from Sigma Aldrich and used as received without any further purification. Silver nitrate and PVP were dissolved in ultra-high pure water (Millipore) at 1:1 by weight. In these conditions,  and
and  are formed due to X-ray induced radiolysis of water that act as reducing agents towards metal-ions resulting in the formation of plasmonic metal nanoparticles. An appropriate irradiation cell
are formed due to X-ray induced radiolysis of water that act as reducing agents towards metal-ions resulting in the formation of plasmonic metal nanoparticles. An appropriate irradiation cell  of polypropylene with kapton cavity has been designed for this experiment.
of polypropylene with kapton cavity has been designed for this experiment.
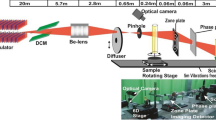
Irradiation was carried out using unfocused monochromatic X-rays beam of energy 20 keV and cross-section area  mm2 at BL-04(imaging beamline, 2.5 GeV storage ring energy and 135 mA maximum current), Indus-2, RRCAT (Indore) INDIA. The photon flux
mm2 at BL-04(imaging beamline, 2.5 GeV storage ring energy and 135 mA maximum current), Indus-2, RRCAT (Indore) INDIA. The photon flux  was
was  ph/s at energy 20 keV 25. Optical layout of BL-04 beamline is shown in the Fig. 1. The expression for integrated dose (Gy) imparted in the sample in such radiation induced experiments was derived as
ph/s at energy 20 keV 25. Optical layout of BL-04 beamline is shown in the Fig. 1. The expression for integrated dose (Gy) imparted in the sample in such radiation induced experiments was derived as

where E = Beam energy, Fph = Photon flux density,  = mass energy-absorption coefficient (% absorption),
= mass energy-absorption coefficient (% absorption),  = Beam cross-sectional area,
= Beam cross-sectional area,  = integrated sample mass exposed to the radiation (exposed cell parameters × sample density) and T = total irradiation time. If the
= integrated sample mass exposed to the radiation (exposed cell parameters × sample density) and T = total irradiation time. If the  lies inside the irradiated cell parameters, the above equation may be written in a simple form:
lies inside the irradiated cell parameters, the above equation may be written in a simple form:

where  = cell thickness and
= cell thickness and  = sample density. Accordingly the energy transferred to the sample is
= sample density. Accordingly the energy transferred to the sample is  Joules/s and the integrated dose for the irradiation time of 01:50 hours comes out to be 62.435 Gy.
Joules/s and the integrated dose for the irradiation time of 01:50 hours comes out to be 62.435 Gy.
Results and Discussions
X-ray irradiation of water produces hydrated electrons  , superoxide
, superoxide  and atom radicals
and atom radicals  , which triggers the reaction by reducing metallic-ions to zero valence silver
, which triggers the reaction by reducing metallic-ions to zero valence silver  as below26:
as below26:




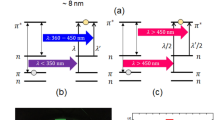
As the zero valent Ag-atoms are formed, particle growth begins to build nanostructure via nucleation process.  might cause the oxidation of
might cause the oxidation of  to
to  , but the probability of oxidation is rare in comparison to reduction because of the large amount of
, but the probability of oxidation is rare in comparison to reduction because of the large amount of  ,
,  and
and  reducing agents than
reducing agents than  . The two major events has been illustrated which are responsible for the formation of small and bigger particles as shown in Fig. 2. Number of
. The two major events has been illustrated which are responsible for the formation of small and bigger particles as shown in Fig. 2. Number of  coalesce to form the nanostructure
coalesce to form the nanostructure  , which further interacts with the
, which further interacts with the  atom and synthesize the AgNP. If
atom and synthesize the AgNP. If  interact with
interact with  nanostructure, it results in the growth
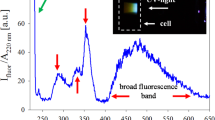
nanostructure, it results in the growth  of AgNP with larger size. The colour phase transition from transparent white to yellow during the X-rays irradiation is attributed to the LSPR27 of AgNP and is the first confirmation towards the formation of nanoparticles. After the irradiation, product solution with appropriate dilution was characterized by the UV-Visible photo-spectrometer (JASCO V-630, USA). The UV-visible spectra of product sample after irradiation of aqueous solution of AgNO3 + PVP (d1) and AgNO3 + PVP + isopropanol (A5) along-with the fitted curve is shown in the Fig. 3. The absorption band (422 and 406 nm) of both the samples lies in the characteristic range27 (380–430 nm) of AgNP and confirmed the formation of same.
of AgNP with larger size. The colour phase transition from transparent white to yellow during the X-rays irradiation is attributed to the LSPR27 of AgNP and is the first confirmation towards the formation of nanoparticles. After the irradiation, product solution with appropriate dilution was characterized by the UV-Visible photo-spectrometer (JASCO V-630, USA). The UV-visible spectra of product sample after irradiation of aqueous solution of AgNO3 + PVP (d1) and AgNO3 + PVP + isopropanol (A5) along-with the fitted curve is shown in the Fig. 3. The absorption band (422 and 406 nm) of both the samples lies in the characteristic range27 (380–430 nm) of AgNP and confirmed the formation of same.
In the previous studies, isopropanol or ethanol had been introduced as a  scavenger to avoid the oxidation of metal nanoparticles13. In our study, we have performed the experiment with and without the presence of this scavenger and investigate the actual mechnism. We did not observe any oxidation, which might be due to the presence of PVP that has enough capability to cap the particles and prevent them from the oxidation. Hence the presence of isopropanol/ethanol not only scavenges from oxidation, but also produces the reducing agent which reduce the size of nanoparticles. The centroid (peak-position) and the broadening (FWHM) of UV-vis spectra of AgNP depend upon the particle size, shape and surface charge28. We have fitted/simulated the data according to the extended version of “Mie-Theory”28 to estimate the size of nanoparticles so formed. According to Mie-theory, particle size of samples d1 and A5 with absorption centroid at 422 and 406 nm, band width of 135.48 and 105.17 nm comes out to be 2.69 and 4.16 nm respectively (Table 1). Figure 4 shows the TEM micrographs of both the samples along-with the histograms. TEM micrographs were investigated by the imageJ package and data is fitted using Gaussian function. The calculated average size of NP for the sample d1 and A5 are 3.61 and 4.00 nm respectively. The histogram shows that the maximum intensity of the nanoparticles present in the solution is in agreement with the size estimation by extended version of Mie-theory (Table 1). To further check the self-lifetime of synthesized plasmonic nanoparticles, TEM measurements were repeated after the time span of 25 days and it is clear that there is no coagulation of nanoparticles as shown in Fig. 4. Synthesized nanoparticles have ultrahigh stability and retain the same size and shape profile as of freshly synthesized even after the age of 68 days (Table 1), as shown in the Fig. 5. A small decrement as observed in the average particle size is because of centrifugation of the samples, result in the highly concentrated product sample (clearly revealed in Fig. 4) and lead to collect the smaller particles also by TEM. In addition to this, the role of X-ray energy has been investigated by exposing the sample to different energies at constant irradiation dose. As the X-ray energy increases, the sample becomes more transparent to corresponding beam resulting in loss of irradiation dose. So the energy-time product has been set to keep the irradiation dose constant. Figure 6 shows the UV-Vis spectra of samples irradiated at different energies reveals that high X-ray energy produces the “hot” hydrated electron (although the dose is constant), which reduces the metal salt with inflate speed resulting in enhancement of particle size. The process of generation of “hot”
scavenger to avoid the oxidation of metal nanoparticles13. In our study, we have performed the experiment with and without the presence of this scavenger and investigate the actual mechnism. We did not observe any oxidation, which might be due to the presence of PVP that has enough capability to cap the particles and prevent them from the oxidation. Hence the presence of isopropanol/ethanol not only scavenges from oxidation, but also produces the reducing agent which reduce the size of nanoparticles. The centroid (peak-position) and the broadening (FWHM) of UV-vis spectra of AgNP depend upon the particle size, shape and surface charge28. We have fitted/simulated the data according to the extended version of “Mie-Theory”28 to estimate the size of nanoparticles so formed. According to Mie-theory, particle size of samples d1 and A5 with absorption centroid at 422 and 406 nm, band width of 135.48 and 105.17 nm comes out to be 2.69 and 4.16 nm respectively (Table 1). Figure 4 shows the TEM micrographs of both the samples along-with the histograms. TEM micrographs were investigated by the imageJ package and data is fitted using Gaussian function. The calculated average size of NP for the sample d1 and A5 are 3.61 and 4.00 nm respectively. The histogram shows that the maximum intensity of the nanoparticles present in the solution is in agreement with the size estimation by extended version of Mie-theory (Table 1). To further check the self-lifetime of synthesized plasmonic nanoparticles, TEM measurements were repeated after the time span of 25 days and it is clear that there is no coagulation of nanoparticles as shown in Fig. 4. Synthesized nanoparticles have ultrahigh stability and retain the same size and shape profile as of freshly synthesized even after the age of 68 days (Table 1), as shown in the Fig. 5. A small decrement as observed in the average particle size is because of centrifugation of the samples, result in the highly concentrated product sample (clearly revealed in Fig. 4) and lead to collect the smaller particles also by TEM. In addition to this, the role of X-ray energy has been investigated by exposing the sample to different energies at constant irradiation dose. As the X-ray energy increases, the sample becomes more transparent to corresponding beam resulting in loss of irradiation dose. So the energy-time product has been set to keep the irradiation dose constant. Figure 6 shows the UV-Vis spectra of samples irradiated at different energies reveals that high X-ray energy produces the “hot” hydrated electron (although the dose is constant), which reduces the metal salt with inflate speed resulting in enhancement of particle size. The process of generation of “hot”  is dependent on X-rays energy, flux and sample holder size. The particle size and concentration is of utmost level at 20 keV in case of our sample holder dimensions. If, one selects a very thin sample holder then even low energy X-rays could lead to the formation of metallic nanoparticles.
is dependent on X-rays energy, flux and sample holder size. The particle size and concentration is of utmost level at 20 keV in case of our sample holder dimensions. If, one selects a very thin sample holder then even low energy X-rays could lead to the formation of metallic nanoparticles.
The presented results shows the novel synthesis protocol for the production of bulk amount of AgNP under the irradiation of synchrotron monochromatic X-rays. This work also leads to real-time characterization of material which would help to understand the process of crystal growth phenomena and the evolution of electronic structure. This experimental technique delivers the scope for growing the nanoparticles with desired particle size, plasmonic and electronic properties that will definitely enhance the quality of photovoltaic cell. The particle size and reaction rate could be controlled by optimizing the radiation beam energy and dose for the production of high purity grade plasmonic metal nanoparticles. TEM report shows that the synthesized nanomaterial is more polydisperse in comparison with one obtained by the gamma ray irradiation. This is possibly because of the decrease in the radiation penetration depth in the sample due to the formation of nanoparticles by the time. And the second reason is due to the decay of beam current over the time results in decreasing the photon flux. To cover these issues, we plan to fabricate the irradiation cell with circular kepton cavity and uniform rotating mechanism.
Additional Information
How to cite this article: Bharti, A. et al. Monochromatic X-Ray Induced Novel Synthesis of Plasmonic Nanostructure for Photovoltaic Application. Sci. Rep. 6, 22394; doi: 10.1038/srep22394 (2016).
References
Green, M. A. & Pillai, S. Harnessing plasmonics for solar cells. Nature Photonics. 6, 130–132 (2012).
Polyakov, A. et al. Plasmon resonance tuning in metallic nanocavities. Sci. Rep. 2, 933 (2012).
Chirumamilla, M., Gopalakrishnan, A., Toma, A., Zaccaria, R. P. & Krahne, R. Plasmon resonance tuning in metal nanostars for surface enhanced Raman scattering. Nanotechnology. 25, 1–7 (2014).
Singh, S., Bharti, A. & Meena, V. K. Green synthesis of multi-shaped silver nanoparticles: optical, morphological and antibacterial properties. J Mater Sci: Mater Electron. 26, 3638–3648 (2015).
Mallin, M. P. & Murphy, C. J. Solution-Phase Synthesis of Sub-10 nm Au-Ag Alloy Nanoparticles. Nano Lett. 11, 1235–1237 (2002).
Nepijko, S. A., Levlev, D. N., Schulze, W., Urban, J. & Ertl, G. Growth of rod like silver nanoparticles by vapour deposition of small clusters. Chem. Phys. Chem. 3, 140–142 (2000).
Renaud, G. et al. Real-Time Monitoring of Growing Nanoparticles. Science. 300, 1416–1419 (2003).
Belloni, J. et al. Radiation-induced synthesis of mono- and multi-metallic clusters and nanocolloids. New J. Chem. 22, 1239–1255 (1998).
Abedini, A. et al. A review on radiation-induced nucleation and growth of colloidal metallic nanoparticles. Nanoscale Res Lett. 8, 474–483 (2013).
Song, J. H. & Park, J. R. Synthesis of Au and Ag Nanomaterials via Proton Beam Irradiation. J Korean Phys Soc. 5, 2143–2147 (2009).
Bogle, K. A., Dhole, S. D. & Bhoraskar, V. N. Silver nanoparticles: synthesis and size control by electron irradiation. Nanotechnology. 17, 3204–3208 (2006).
Liu, Y., Chen, S., Zhong, L. & Wu, G. Preparation of high stable silver nanoparticle dispersion by using sodium alginate as a stabilizer under gamma radiation. Radiat. Phys. Chem. 78, 251–255 (2009).
Remita, S. et al. X-ray radiolysis induced formation of silver nano-particles: A SAXS and UV-visible absorption spectroscopy study. Nucl. Instrum. Methods Phys. Res., Sect. B. 263, 436–440 (2007).
Remita, S., Fontaine, P., Rochas, C., Muller, F. & Goldmann, M. Radiation induced synthesis of silver nanoshells formed onto organic micelles. Eur. Phys. J. D. 34, 231–233 (2005).
Hahner, G. Near edge X-ray absorption fine structure spectroscopy as a tool to probe electronic and structural properties of thin organic films and liquids. Chem. Soc. Rev. 35, 1244–1255 (2006).
Cheng, N. N. et al. Chemical Enhancement by Nanomaterials under X-ray Irradiation. J. Am. Chem. Soc. 134, 1950–1953 (2012).
Yang, Y. C., Wang, C. H., Hwu, Y. K. & Je, J. H. Synchrotron X-ray synthesis of colloidal gold particles for drug delivery. Mater. Chem. Phys. 100, 72–76 (2006).
Lin, K. N. et al. A novel method of supporting gold nanoparticles on MWCNTs: Synchrotron X-ray reduction. China Particuology. 5, 237–241 (2007).
Ma, Q., Moldovan, N., Mancini, D. C. & Rosenberg, R. A. Synchrotron-radiation-induced, selective-area deposition of gold on polyimide from solution. Appl. Phys. Lett. 15, 2014–2016 (2000).
Wang, C. L. et al. One-pot synthesis of AuPt alloyed nanoparticles by intense x-ray irradiation. Nanotechnology 22, 065605 (2011).
Kim, C. C. et al. X-ray synthesis of nickel-gold composite nanoparticles. Mater. Chem. Phys. 100, 292–295 (2006).
Georg, H. Near edge X-ray absorption fine structure spectroscopy as a tool to probe electronic and structural properties of thin organic films and liquids. Chem. Soc. Rev. 35, 1244–1255 (2006).
Arvid, P. L. et al. High-throughput roll-to-roll X-ray characterization of polymer solar cell active layers. J. Mater. Chem. 22, 22501–22509 (2012).
Lee, H. J., Je, J. H., Hwu, Y. & Tsai, W. L. Synchrotron X-ray induced solution precipitation of nanoparticles. Nucl. Instr. and Meth. in Phys. Res. B. 199, 342–347 (2003).
Agrawal, A. K. et al. Design, development and first experiments on the X-ray imaging beamline at Indus-2 synchrotron source RRCAT, India. J. Synchrotron Rad. 22, 1531–1539 (2015).
Muller, F. et al. Synthesis of Nanostructured Metal-Organic Films: Surface X-ray Radiolysis of Silver Ions Using a Langmuir monolayer as a template. Langmuir. 20, 4791–4794 (2004).
Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir. 12, 788–800 (1996).
Petit, C., Lixon, P. & Pileni, M. P. In-situ synthesis of silver nanocluster in AOT reverse micelles. J. Phys. Chem. 97, 12974–12983 (1993).
Acknowledgements
The authors are thankful for the supports by Dr. Amar Sinha (NXPD, BARC), Dr. Ravindra D Makde (RRCAT) and Mr. Balwant Singh (BL-04, RRCAT) along-with Indus-II staff during the experiment. Financial support for publication fee by Panjab University, Chandigarh (Research Promotion Cell, UGC XII<sup>th</sup> plan) is duly acknowledged. S.G. acknowledges partial supports from UGC-BSR and UGC-DAE CSR (2014-15/1204) Research Project. SG also acknowledges the support from Dr. VR Reddy and Prof. SN Jha under UGC-DAE CSR project.
Author information
Authors and Affiliations
Contributions
A.B. conceived the experiments, A.B., R.B., S.G. and A.A. conduct the experiments and A.B., R.B., S.G. and N.G. analyzed the results. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Bharti, A., Bhardwaj, R., Agrawal, A. et al. Monochromatic X-Ray Induced Novel Synthesis of Plasmonic Nanostructure for Photovoltaic Application. Sci Rep 6, 22394 (2016). https://doi.org/10.1038/srep22394
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep22394
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.