Abstract
While glucose is the fundamental source of energy in most eukaryotes, it is not always abundantly available in natural environments, including within the human body. Eukaryotic cells are therefore thought to possess adaptive mechanisms to survive glucose-limited conditions, which remain unclear. Here, we report a novel mechanism regulating cell cycle progression in response to abrupt changes in extracellular glucose concentration. Upon reduction of glucose in the medium, wild-type fission yeast cells undergo transient arrest specifically at G2 phase. This cell cycle arrest is dependent on the Wee1 tyrosine kinase inhibiting the key cell cycle regulator, CDK1/Cdc2. Mutant cells lacking Wee1 are not arrested at G2 upon glucose limitation and lose viability faster than the wild-type cells under glucose-depleted quiescent conditions, suggesting that this cell cycle arrest is required for extension of chronological lifespan. Our findings indicate the presence of a novel cell cycle checkpoint monitoring glucose availability, which may be a good molecular target for cancer therapy.
Similar content being viewed by others
Introduction
Cell growth, division and differentiation in eukaryotes are regulated by various compounds in the microenvironment surrounding the cells, such as growth factors and nutrients. In unicellular eukaryotes, nutrients in the medium are major determinants of the timing of cell division (i.e., cell cycle progression) and differentiation. In the fission yeast, Schizosaccharomyces pombe, for example, the depletion of nitrogen sources accelerates cell cycle progression temporarily and then arrests the cells in G1 phase after two rounds of cell division1,2,3. S. pombe cells subsequently undergo sexual differentiation in the presence of mating pheromones, whereas the cells enter the quiescent (G0) state in their absence, which confers resistance to various types of stress4,5. In multicellular organisms, such as fruit flies, the availability of nutrients during larval development determines the size of the body by modulating the sizes and the numbers of cells via nutrient-sensing signalling cascades involving the target of rapamycin (TOR) kinase and insulin-like growth factors6,7,8. Thus, in both unicellular and multicellular eukaryotes, the rate and the timing of cell cycle progression are regulated in response to changes in extracellular nutritional status.
The TOR kinases, which form two distinct complexes, TORC1 and TORC2, are suggested to play a pivotal role in cellular response to extracellular nutrients, such as amino acids9,10,11,12,13. In S. pombe, the TOR complexes control cell growth, division and sexual differentiation in response to limitation of nitrogen sources and/or glucose14,15,16,17,18,19,20,21,22,23,24,25. Very recently, we demonstrated that S. pombe TORC2, but not TORC1, is required for proper localization of the high-affinity glucose transporter, Ght5, transcription of which is elevated upon glucose restriction in a manner dependent on calcium/calmodulin dependent kinase kinase (CaMKK)26,27. In S. pombe, TORC2 and CaMKK are required for enhancement of glucose uptake and thus for vigorous cell proliferation, under glucose-limited conditions. Intriguingly, when S. pombe cells are transferred from high-glucose (111 mM) medium to low-glucose (4.4 mM) medium, they stop dividing transiently before resuming rapid proliferation26,28. These findings indicate that reduction of extracellular glucose triggers large-scale remodelling in the molecular machinery involved in regulation of glucose transport and metabolism and cell proliferation.
Periodic activation and inactivation of cyclin-dependent protein kinases (CDKs) drive the progression of the cell cycle in eukaryotes. While higher eukaryotes possess multiple types of CDK, each of which is responsible for transitions of different stages of the cell cycle, the single CDK (Cdc2/CDK1), which is thought to be the prototype of the CDKs, controls the entire cell cycle in S. pombe, in association with different stage-specific cyclins29,30. Studies using various model organisms, including fission yeast, have revealed the core mechanism underlying the regulation of cell cycle progression; the activities of CDKs are regulated by association with the cyclin subunits and phosphorylation. The evolutionarily conserved tyrosine kinase(s) and the counteracting phosphatase, Wee1 (and a related kinase, Mik1) and Cdc25, which were originally identified in fission yeast, control the activity of Cdc2/CDK1 through the inhibitory phosphorylation of its tyrosine 15 (Tyr 15) residue31,32,33,34,35,36. Tyr 15 phosphorylation by Wee1 inactivates Cdc2 and prevents the G2/M transition, while its dephosphorylation by Cdc25 promotes entry into M phase. Thus, the balance between Wee1 and Cdc25 determines the timing of the onset of M phase.
Cell cycle progression is regulated in response to various types of stress. Genotoxic stresses, such as DNA damage and incomplete replication, activate the checkpoint, which prevents cell cycle progression until the stresses are removed37,38,39. In response to these genotoxic stresses, the evolutionarily conserved DNA structure checkpoint signalling cascade, upstream of which ATM (Ataxia telangiectasia mutated) and ATR (ATM and Rad3-related) kinases act, ultimately inhibit Cdc25, so that the cells are arrested in G2 phase. Defects in the checkpoints result in the rapid loss of genomic integrity and cell viability in the presence of genotoxic stresses. In contrast, the molecular mechanism underlying the modulation of cell cycle progression in response to nutritional stresses, such as glucose restriction, is largely unknown and the physiological significance of such a mechanism, if it exists, remains to be elucidated.
Here, we explore the mechanism underlying the regulation of cell cycle progression under low glucose conditions and show that glucose restriction causes transient G2 arrest in a manner dependent on Wee1 kinase. Mutant cells lacking Wee1 failed to cease cell cycle progression at G2 phase upon glucose restriction and lost cell viability after glucose depletion. Our findings indicate that Wee1-dependent G2 arrest is a novel checkpoint control in response to glucose restriction, which extends the chronological lifespan under conditions of glucose depletion.
Results
Glucose restriction causes transient cell cycle arrest in G2 phase
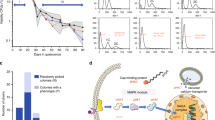
While regular laboratory media for S. pombe contain 2–3% (111–167 mM) glucose, the wild-type (WT) cells proliferate in medium containing only 0.08% (4.4 mM) glucose, which is equivalent to that in normal human blood, at a division rate similar to that in regular high-glucose medium. When transferred from high-glucose (2%, 111 mM) to low-glucose (0.08%, 4.4 mM) medium, cells stop dividing for a period of 1–2 generations (3–5 hours at 26 °C) and then resume vigorous cell division in a manner dependent on full mitochondrial function26,27,28,40. To gain mechanistic insight into cell division control in response to limitation of extracellular glucose, we monitored cell cycle progression in WT cells transferred from high-glucose to low-glucose medium by measuring the proportion of cells with a septum (septation index, %SI), which is a useful hallmark of cytokinesis (Fig. 1A). While %SI was maintained at ~15% in an asynchronous population of cells growing in synthetic Edinburgh minimal medium 2 (EMM2 medium) containing a high glucose concentration (111 mM) at 26 °C, it dropped to 2.2% at 2 hours after transfer to low-glucose (4.4 mM) EMM2 medium. The %SI then returned to a level comparable to that in high-glucose medium, as the cell number resumed increasing at a rate of 3.8 hours per division, which was virtually identical to the rate in high-glucose medium28. This observation indicated that acute restriction of extracellular glucose caused transient cell cycle arrest before the onset of cytokinesis. Notably, the length of cells did not increase after the shift to low-glucose medium, but rather became shorter, suggesting that cell growth (i.e., the extension of cell length) was inhibited during this arrest caused by glucose restriction, unlike cell cycle arrest due to stresses causing DNA damage and/or incomplete DNA replication, even in the presence of which the WT cells continued to grow41,42.
Glucose restriction causes transient cell cycle arrest in G2 phase.
(A) Time courses of septation index (upper panel) and relative cell density (lower panel) of WT fission yeast cells that were transferred from high-glucose (111 mM) to low-glucose (4.4 mM) medium at time = 0 were examined. The averages of three independent experiments are shown and the bars represent standard deviation (SD). (B) The distributions of cellular DNA content were measured in WT cells by flow cytometry analysis after transfer from high-glucose to low-glucose medium at time = 0. The positions of the peak of 1C (before replication) and 2C (after replication) DNA cells are indicated by arrows. (C) The time course of changes in the proportion of cells with a mitotic spindle (spindle index) was examined in WT cells after transfer to low-glucose medium. The experiments were repeated independently three times and the averages and SD are shown. (D) Fluorescence microscopic images of WT cells expressing GFP-fused α-tubulin and Sid4 before (left panel) and 2 hours after transfer to low-glucose medium (right panel) are shown. GFP fluorescence is pseudocoloured green, whereas the nuclear DNA stained by DAPI is shown in magenta. Bars, 5 μm.
To determine in which phase of the cell cycle the cells were transiently arrested upon glucose restriction, we performed flow cytometry analysis to measure cellular DNA content (Fig. 1B). The histograms showed the distribution of the DNA content per cell before/after transfer from high-glucose to low-glucose medium. A single peak appeared before transfer (time = 0 hour), as most WT S. pombe cells growing asynchronously in regular high-glucose EMM2 medium are in G243,44. After transfer to low-glucose medium (time = 1–6 hours), only one peak at the 2C DNA content was still present; even at the time point when %SI became minimal (time = 2 hours), no other sub-peaks appeared, indicating that transient cell cycle arrest due to glucose restriction occurred after the completion of DNA replication, i.e., in G2 or M phase. We then measured the proportion of cells with mitotic spindle (spindle index, %SpI) after transfer to low-glucose medium using a WT strain expressing α-tubulin and Sid4 labelled with green fluorescent protein (GFP), which allowed visualization of the spindle microtubules and poles, respectively (Fig. 1 C,D). The %SpI, which was 6.5% before transfer, dropped to nearly 0% transiently at 1–2 hours after transfer to low-glucose medium and then recovered to the same level as before the shift at 4 hours (Fig. 1C). The absence of cells with a mitotic spindle at 2 hours indicated that the cells were arrested before the onset of M phase. Taken together, these observations suggested that glucose restriction caused temporary arrest specifically at G2 phase. It should be noted that this G2 arrest was not caused by hypotonic shock due to glucose restriction; %SI still dropped after transfer to low-glucose medium supplemented with 107 mM sorbitol, which compensated the reduction of osmotic pressure (Supplementary Fig. 1).
Wee1 is required for transient G2 arrest due to glucose restriction
To identify genes required for cell proliferation specifically under glucose-limited conditions, we systematically screened a S. pombe gene deletion strain library for mutant strains defective in cell proliferation on low-glucose medium. Approximately 150 gene deletion mutant strains were identified, including six mutants lacking a cycle-related gene (mcl1+, mpr1+, rad1+, rad26+, sum2+ or wee1+)27. To examine whether these six genes were involved in transient G2 arrest upon glucose restriction, %SI was measured in mutant cells lacking each one of these cell cycle-related genes after transfer to low-glucose medium. Among these genes, the wee1+ gene was suggested to be essential for cell cycle control in response to glucose restriction; in mutant cells lacking wee1+ (wee1Δ), %SI remained high after transfer to low-glucose medium, although the cell number stopped increasing transiently (Fig. 2A). This result suggested that, when the extracellular glucose concentration was reduced abruptly, the wee1Δ cells ceased dividing at any cell cycle stage presumably due to energy (i.e., ATP) shortage. Consistent with this suggestion, %SpI representing the proportion of mitotic cells remained high in the wee1Δ mutant even after transfer to low-glucose medium and cells in early- to late-mitosis were frequently observed in wee1Δ (Fig. 2B,C), in marked contrast to WT controls in which mitotic cells were seldom observed at 1–2 hours after transfer to low-glucose medium (Fig. 1C,D). Measurement of cellular DNA content revealed that 1C DNA cells became prominent in wee1Δ after prolonged incubation in low-glucose medium (Fig. 2D), indicating that cells before the onset of DNA replication, completion of which requires a large amount of ATP, were gradually accumulated in this mutant strain during cultivation under glucose-limited conditions. Taken together, these observations indicated that Wee1 is essential for G2 cell cycle arrest in response to glucose restriction.
Mutant cells lacking wee1+ failed to be arrested at G2 phase after glucose restriction.
(A) Time courses of changes in septation index (upper panel) and relative cell density (lower panel) of mutant cells lacking the wee1+ gene (wee1Δ) that were transferred from high-glucose (111 mM) to low-glucose (4.4 mM) medium at time = 0 were examined. The averages of three independent experiments are shown and the bars indicate ±SD. (B) The time course of changes in the spindle index was examined in wee1Δ cells after transfer to low-glucose medium. The experiments were repeated independently three times and the averages ±SD are shown. (C) Fluorescence microscopic images of wee1Δ cells expressing GFP-fused α-tubulin before (upper panel) and 2 hours after transfer to low-glucose medium (lower panel) are shown. GFP fluorescence is pseudocoloured green, whereas the nuclear DNA stained by DAPI is shown in magenta. Bars, 5 μm. (D) The distributions of cellular DNA content were measured in wee1Δ cells by flow cytometry analysis after transfer from high-glucose to low-glucose medium at time = 0. The positions of the peak of 1C (before replication) and 2C (after replication) DNA cells are indicated by arrows.
To confirm the observations above, %SI was measured in wee1-50 cells, which harbour a temperature-sensitive mutation in the wee1+ gene. At a restrictive temperature, 36 °C, the wee1-50 mutant cells divide at a reduced cell size due to inactivation of Wee1 leading to premature entry into M phase45. Transient reduction of %SI was not observed in the wee1-50 mutant after transfer to low-glucose medium even at a permissive temperature, 26 °C, where the cell length appeared largely normal, suggesting that fully functional Wee1 is required for G2 cell cycle arrest upon glucose restriction (Fig. 3).
Fully functional Wee1 is required for G2 cell cycle arrest after glucose restriction.
Time courses of changes in septation index (upper panel) and cell density (lower panel) of wee1-50 mutant cells that were transferred from high-glucose (111 mM) to low-glucose (4.4 mM) medium at time = 0 were examined. Cells were cultivated at 26 °C, a permissive temperature for the wee1-50 mutant, throughout the measurement. For %SI, 200 cells were examined.
Wee1 is essential for extension of chronological lifespan under conditions of glucose starvation
The above findings strongly suggest the existence of a novel cell cycle control mechanism involving Wee1, which blocks G2/M transition temporarily in response to abrupt reduction of extracellular glucose concentration from 111 mM to 4.4 mM. This raises questions regarding the physiological consequence of the transient G2 arrest upon glucose restriction. In nature, glucose is thought to be exhausted shortly after its reduction from the environment of the cells and therefore we suspect that this arrest may be required for cellular adaptation to glucose-limited environments and preparation for survival in the complete absence of glucose. WT S. pombe cells, which were transferred directly from high-glucose (111 mM) medium to medium completely lacking glucose (glucose-depleted medium), lost viability within 3 days28 (Fig. 4A), whereas they could maintain high viability for more than 2 weeks in glucose-depleted medium if they were cultivated in low-glucose (4.4 mM) medium for 1 day in prior to transfer28 (Fig. 4B). In contrast, wee1Δ mutant cells lost viability faster than WT in glucose-depleted medium even after pre-cultivation in low-glucose medium (Fig. 3B); at 14 days after transfer from high-glucose medium, the viabilities of WT and wee1Δ cells pre-incubated in low-glucose medium before transfer were 68.8% and 20.4%, respectively. These results indicated that adaptation to low-glucose environments greatly extended the cellular chronological lifespan under conditions of glucose depletion and that Wee1 plays a pivotal role in this lifespan extension.
Cultivation in low-glucose medium extends chronological lifespan under glucose starvation in a Wee1-dependent manner.
(A,B) Time course of changes in viability of cells cultivated in glucose-depleted medium. WT (open circles) or wee1Δ (crosses) cells grown in high-glucose (111 mM) medium were transferred to medium lacking glucose directly (A) or after 1-day incubation in low-glucose (4.4 mM) medium (B). Aliquots of the cell culture were taken at the indicated time points and viability was determined by measuring the proportion of cells forming colonies on solid YES medium. (C) Passage of transient G2-arrest period in low glucose is essential for extension of lifespan under conditions of glucose starvation. The experimental procedure is illustrated in the upper panel. The WT cells grown in high-glucose (111 mM) medium were transferred to and cultivated in low-glucose (4.4 mM) medium for 0, 0.5, 3, 6 or 12 hours and then transferred to medium without glucose. Cell viability was measured after 7-day incubation in glucose-depleted medium. The averages of three independent experiments are shown. The bars indicate ±SD.
We next examined the minimum length of pre-incubation in low-glucose medium required for extension of chronological lifespan under conditions of glucose depletion. WT cells that were pre-incubated in low (4.4 mM)-glucose medium for 0.5, 3, 6 and 12 hours were transferred to glucose-depleted medium and their viability was measured after 7 days of cultivation without glucose (Fig. 4C). While cells pre-incubated in low-glucose medium for longer than 3 hours retained high viability (~70%), the viability of cells pre-incubated for only 0.5 hour decreased to 6.9%. This result suggested that the full extension of chronological lifespan requires pre-incubation in low-glucose medium for more than 3 hours. Notably, reduction of %SI due to G2 arrest was hardly perceptible at 0.5 hour after glucose restriction and became most prominent at 2 hours. Therefore, the passage of G2 cell cycle arrest period in low-glucose medium, during which cells supposedly acclimate to glucose-limited environments, may be important for extension of cellular lifespan.
Discussion
Cell cycle progression is regulated in response to changes in environmental conditions. Our findings strongly suggest the presence of a cell cycle regulatory mechanism that temporarily blocks G2/M transition in response to reduction of extracellular glucose supply. Transient reduction of %SpI and %SI indicates that S. pombe WT cells in M phase at the moment of transfer to low-glucose medium complete nuclear division and subsequent cytokinesis before stopping proliferation, whereas the cells in G2 phase do not initiate mitosis. Cells in G1 or S phase appear to finish DNA replication before cell cycle arrest, as the majority of cells contain 2C DNA after glucose restriction. This G2 block due to glucose restriction is thought to be determined genetically, as deletion of the wee1+ gene, which encodes an evolutionarily conserved tyrosine kinase regulating CDK activities, bypasses this G2 block and causes the accumulation of cells with unreplicated DNA during proliferation under glucose-limited conditions. We propose that this cell cycle regulatory mechanism is a novel type of checkpoint, which monitors glucose availability and allows cells to adapt to glucose-limited environments. During the period of G2 arrest, cells may enhance their capability for glucose transport and mitochondrial ATP generation and gain energy and carbon source sufficient for completion of mitosis and cytokinesis. This glucose-monitoring checkpoint may, at least in part, extend the cellular chronological lifespan in the absence of glucose, as mutant cells lacking the wee1+ gene have a shorter lifespan than WT cells. Although further studies are required to determine the mechanism underlying sensing of the amount of glucose uptake, we suspect that the CaMKK (Ssp1 in S. pombe) – Protein phosphatase type 6 (PP6, Ppe1 in S. pombe) signalling cascade may play a pivotal role in this glucose-monitoring checkpoint. We reported previously that S. pombe Ssp1 and the PP6-inhibitor, Sds23, which genetically interacts with Ssp146, are essential for transcriptional elevation of the high-affinity glucose transporter gene, ght5+, upon glucose limitation26. While the Ssp1 protein is localized mainly in the cytoplasm, it moves transiently to the vicinity of the cell surface when cells are exposed to stresses, such as an osmotic stress47. The CaMKK signalling pathway may transmit molecular signals from an as yet unidentified glucose sensor on the cell surface to cell cycle regulators, including Wee1, as well as the transcriptional regulator, Scr126.
It is noteworthy that deletion of the wee1+ gene shortens the chronological lifespan of S. pombe cells under glucose-depleted conditions, whereas it does not substantially affect the viability of the cells growing in glucose-rich medium. Although it remains to be determined whether the “glucose-monitoring checkpoint” described above exists in higher eukaryotes, if present, inhibition of Wee1 activity in these organisms may selectively kill cells under conditions of glucose starvation. As microenvironments surrounding tumour cells are likely to contain only a limited supply of nutrients, including glucose, these cells may be selectively killed by treatment capable of inhibiting Wee1 kinase. Therefore, our findings suggest that Wee1 is a good molecular target for cancer therapy.
Methods
General techniques and strains
General procedures for handling of S. pombe were described previously26. For cultivation of S. pombe cells, rich yeast extract/glucose/supplements (YES) medium and synthetic minimal EMM2 medium were used with modified glucose concentrations as indicated48. Unless otherwise stated, the cells were cultivated at 26 °C. Cell viability was expressed as the ratio of the number of colonies formed on YES solid medium containing 167 mM (3%) glucose to the total number (~1,000) of cell bodies plated. Measurement of cellular DNA content by flow cytometry was performed as described previously49. For C-terminal gene tagging and gene disruption, the PCR-mediated method50 was employed. The strains used in this study were WT (972; h−), wee1Δ (SP4892; h− wee1Δ::NAT), WT expressing GFP-labelled α-tubulin and Sid4 (K399; h− lys1+::nda2Prom-GFP-atb2 sid4-GFP::KanMX4) and wee1Δ expressing GFP-labelled α-tubulin and Sid4 (K391; h+ wee1Δ::NAT lys1+::nda2Prom-GFP-atb2 sid4-GFP::KanMX4).
Fluorescence microscopy
Fluorescence microscopy was performed using an EVOS fl microscope system (ThermoFisher Scientific, Waltham, MA, USA) equipped with 100× (numerical aperture (NA) 1.35) and 20× (NA 0.45) objective lenses, or an Axiovert 200M microscope system (Carl Zeiss, Oberkochen, Germany) equipped with a 100× objective lens (NA 1.40). Calcofluor (10 μg/mL; Sigma-Aldrich, St. Louis, MO, USA) and 4′,6-diamidino-2-phenylindole (DAPI, 50 μg/mL) were applied to cells fixed with 2.5% glutaraldehyde51 for fluorescent staining of DNA and septum, respectively. To measure %SI, 400 cells were examined in each sample, unless otherwise stated. To visualize microtubules and SPB, cells expressing GFP-labelled α-tubulin and Sid4 were harvested by vacuum filtration and fixed by immersing in methanol chilled to –80 °C for 30 minutes. The fixed cells were washed with phosphate buffered saline three times before observation. To measure %SpI, 200 cells were examined in each sample.
Additional Information
How to cite this article: Masuda, F. et al. Glucose restriction induces transient G2 cell cycle arrest extending cellular chronological lifespan. Sci. Rep. 6, 19629; doi: 10.1038/srep19629 (2016).
References
Sajiki, K. et al. Genetic control of cellular quiescence in S. pombe. J Cell Sci 122, 1418–1429 (2009).
Shimanuki, M. et al. Two-step, extensive alterations in the transcriptome from G0 arrest to cell division in Schizosaccharomyces pombe. Genes Cells 12, 677–692 (2007).
Young, P. G. & Fantes, P. A. Schizosaccharomyces pombe mutants affected in their division response to starvation. Journal of Cell Science 88, 295–304 (1987).
Costello, G., Rodgers, L. & Beach, D. Fission yeast enters the stationary phase G0 state from either mitotic G1 or G2. Curr Genet 10, 119–125 (1986).
Su, S. S., Tanaka, Y., Samejima, I., Tanaka, K. & Yanagida, M. A nitrogen starvation-induced dormant G0 state in fission yeast: the establishment from uncommitted G1 state and its delay for return to proliferation. J Cell Sci 109 (Pt 6), 1347–1357 (1996).
Colombani, J. et al. A nutrient sensor mechanism controls Drosophila growth. Cell 114, 739–749 (2003).
Hietakangas, V. & Cohen, S. M. Regulation of tissue growth through nutrient sensing. Annu Rev Genet 43, 389–410, doi: 10.1146/annurev-genet-102108-134815 (2009).
Layalle, S., Arquier, N. & Léopold, P. The TOR pathway couples nutrition and developmental timing in Drosophila. Dev Cell 15, 568–577, doi: 10.1016/j.devcel.2008.08.003 (2008).
Ikai, N., Nakazawa, N., Hayashi, T. & Yanagida, M. The reverse, but coordinated, roles of Tor2 (TORC1) and Tor1 (TORC2) kinases for growth, cell cycle and separase-mediated mitosis in Schizosaccharomyces pombe. Open Biol 1, 110007 (2011).
Loewith, R. et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10, 457–468 (2002).
Schmelzle, T. & Hall, M. N. TOR, a central controller of cell growth. Cell 103, 253–262 (2000).
Wullschleger, S., Loewith, R. & Hall, M. N. TOR signaling in growth and metabolism. Cell 124, 471–484 (2006).
Yanagida, M., Ikai, N., Shimanuki, M. & Sajiki, K. Nutrient limitations alter cell division control and chromosome segregation through growth-related kinases and phosphatases. Philos Trans R Soc Lond B Biol Sci 366, 3508–3520 (2011).
Cohen, A., Kupiec, M. & Weisman, R. Glucose activates TORC2-Gad8 protein via positive regulation of the cAMP/cAMP-dependent protein kinase A (PKA) pathway and negative regulation of the Pmk1 protein-mitogen-activated protein kinase pathway. J Biol Chem 289, 21727–21737, doi: 10.1074/jbc.M114.573824 (2014).
Hartmuth, S. & Petersen, J. Fission yeast Tor1 functions as part of TORC1 to control mitotic entry through the stress MAPK pathway following nutrient stress. J Cell Sci 122, 1737–1746, doi: 10.1242/jcs.049387 (2009).
Hatano, T., Morigasaki, S., Tatebe, H., Ikeda, K. & Shiozaki, K. Fission yeast Ryh1 GTPase activates TOR Complex 2 in response to glucose. Cell Cycle 14, 848–856, doi: 10.1080/15384101.2014.1000215 (2015).
Hayashi, T. et al. Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes Cells 12, 1357–1370 (2007).
Kawai, M. et al. Fission yeast Tor1 functions in response to various stresses including nitrogen starvation, high osmolarity and high temperature. Curr Genet 39, 166–174 (2001).
Laor, D., Cohen, A., Kupiec, M. & Weisman, R. TORC1 Regulates Developmental Responses to Nitrogen Stress via Regulation of the GATA Transcription Factor Gaf1.MBio 6, e00959, doi: 10.1128/mBio.00959-15 (2015).
Matsuo, T., Kubo, Y., Watanabe, Y. & Yamamoto, M. Schizosaccharomyces pombe AGC family kinase Gad8p forms a conserved signaling module with TOR and PDK1-like kinases. Embo J 22, 3073–3083 (2003).
Matsuo, T., Otsubo, Y., Urano, J., Tamanoi, F. & Yamamoto, M. Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol Cell Biol 27, 3154–3164 (2007).
Petersen, J. & Nurse, P. TOR signalling regulates mitotic commitment through the stress MAP kinase pathway and the Polo and Cdc2 kinases. Nat Cell Biol 9, 1263–1272, doi: 10.1038/ncb1646 (2007).
Shinozaki-Yabana, S., Watanabe, Y. & Yamamoto, M. Novel WD-repeat protein Mip1p facilitates function of the meiotic regulator Mei2p in fission yeast. Mol Cell Biol 20, 1234–1242 (2000).
Shiozaki, K. Nutrition-minded cell cycle. Sci Signal 2, pe74, doi: 10.1126/scisignal.296pe74 (2009).
Weisman, R., Roitburg, I., Nahari, T. & Kupiec, M. Regulation of leucine uptake by tor1+ in Schizosaccharomyces pombe is sensitive to rapamycin. Genetics 169, 539–550 (2005).
Saitoh, S. et al. Mechanisms of expression and translocation of major fission yeast glucose transporters regulated by CaMKK/phosphatases, nuclear shuttling and TOR. Mol Biol Cell 26, 373–386, doi: 10.1091/mbc.E14-11-1503 (2015).
Saitoh, S. & Yanagida, M. Does a shift to limited glucose activate checkpoint control in fission yeast? FEBS Lett 588, 2373–2378, doi: 10.1016/j.febslet.2014.04.047 (2014).
Pluskal, T., Hayashi, T., Saitoh, S., Fujisawa, A. & Yanagida, M. Specific biomarkers for stochastic division patterns and starvation-induced quiescence under limited glucose levels in fission yeast. Febs J 278, 1299–1315 (2011).
Moser, B. A. & Russell, P. Cell cycle regulation in Schizosaccharomyces pombe. Curr Opin Microbiol 3, 631–636 (2000).
Simanis, V. & Nurse, P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell 45, 261–268 (1986).
Gould, K. L. & Nurse, P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature 342, 39–45, doi: 10.1038/342039a0 (1989).
Lundgren, K. et al. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell 64, 1111–1122 (1991).
Parker, L. L. et al. Cyclin promotes the tyrosine phosphorylation of p34cdc2 in a wee1+ dependent manner. EMBO J 10, 1255–1263 (1991).
Russell, P., Moreno, S. & Reed, S. I. Conservation of mitotic controls in fission and budding yeasts. Cell 57, 295–303 (1989).
Russell, P. & Nurse, P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell 45, 145–153 (1986).
Russell, P. & Nurse, P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell 49, 559–567 (1987).
Hartwell, L. H. & Weinert, T. A. Checkpoints: controls that ensure the order of cell cycle events. Science 246, 629–634 (1989).
Russell, P. Checkpoints on the road to mitosis. Trends Biochem Sci 23, 399–402 (1998).
Weinert, T. A. & Hartwell, L. H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science 241, 317–322 (1988).
Takeda, K., Starzynski, C., Mori, A. & Yanagida, M. The critical glucose concentration for respiration-independent proliferation of fission yeast, Schizosaccharomyces pombe. Mitochondrion 22, 91–95, doi: 10.1016/j.mito.2015.04.003 (2015).
al-Khodairy, F. & Carr, A. M. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J 11, 1343–1350 (1992).
Rowley, R., Subramani, S. & Young, P. G. Checkpoint controls in Schizosaccharomyces pombe: rad1. EMBO J 11, 1335–1342 (1992).
Carlson, C. R., Grallert, B., Stokke, T. & Boye, E. Regulation of the start of DNA replication in Schizosaccharomyces pombe. Journal of Cell Science 112, 939–946 (1999).
Forsburg, S. L. & Rhind, N. Basic methods for fission yeast. Yeast 23, 173–183, doi: 10.1002/yea.1347 (2006).
Thuriaux, P., Nurse, P. & Carter, B. Mutants altered in the control co-ordinating cell division with cell growth in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet 161, 215–220 (1978).
Hanyu, Y. et al. Schizosaccharomyces pombe cell division cycle under limited glucose requires Ssp1 kinase, the putative CaMKK and Sds23, a PP2A-related phosphatase inhibitor. Genes Cells 14, 539–554 (2009).
Freitag, S. I., Wong, J. & Young, P. G. Genetic and physical interaction of Ssp1 CaMKK and Rad24 14-3-3 during low pH and osmotic stress in fission yeast. Open Biol 4, 130127, doi: 10.1098/rsob.130127 (2014).
Moreno, S., Klar, A. & Nurse, P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194, 795–823 (1991).
Takayama, Y. & Takahashi, K. Differential regulation of repeated histone genes during the fission yeast cell cycle. Nucleic Acids Res 35, 3223–3237, doi: 10.1093/nar/gkm213 (2007).
Krawchuk, M. D. & Wahls, W. P. High-efficiency gene targeting in Schizosaccharomyces pombe using a modular, PCR-based approach with long tracts of flanking homology. Yeast 15, 1419–1427 (1999).
Adachi, Y. & Yanagida, M. Higher order chromosome structure is affected by cold-sensitive mutations in a Schizosaccharomyces pombe gene crm1+ which encodes a 115-kD protein preferentially localized in the nucleus and its periphery. J Cell Biol 108, 1195–1207 (1989).
Acknowledgements
We acknowledge generous support from the Okinawa Institute of Science and Technology Graduate University (OIST) and the MEXT-Supported Program for the Strategic Research Foundation at Private University from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. Shigeaki Saitoh was supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (Grant Number: 24570221). Kojiro Takeda was supported by Special Ordinary Expense Subsidies for Private Universities from MEXT, Japan.
Author information
Authors and Affiliations
Contributions
F.M., M.I., A.M., L.U., K.T. and S.S. performed the experiments. M.Y., K.T. and S.S. participated in experimental design and analysed the results. K.T. and S.S. wrote the manuscript and prepared Figs 1–4. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Masuda, F., Ishii, M., Mori, A. et al. Glucose restriction induces transient G2 cell cycle arrest extending cellular chronological lifespan. Sci Rep 6, 19629 (2016). https://doi.org/10.1038/srep19629
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19629
This article is cited by
-
Highly concentrated trehalose induces prohealing senescence-like state in fibroblasts via CDKN1A/p21
Communications Biology (2023)
-
CD98hc (SLC3A2) sustains amino acid and nucleotide availability for cell cycle progression
Scientific Reports (2019)
-
Bub1 kinase- and H2A phosphorylation-independent regulation of Shugoshin proteins under glucose-restricted conditions
Scientific Reports (2019)
-
CoQ10 production in Schizosaccharomyces pombe is increased by reduction of glucose levels or deletion of pka1
Applied Microbiology and Biotechnology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.