Abstract
The role of soil microbial variables in shaping the temporal variability of soil respiration has been well acknowledged but is poorly understood, particularly under elevated nitrogen (N) deposition conditions. We measured soil respiration along with soil microbial properties during the early, middle and late growing seasons in temperate grassland plots that had been treated with N additions of 0, 2, 4, 8, 16, or 32 g N m−2 yr−1 for 10 years. Representing the averages over three observation periods, total (Rs) and heterotrophic (Rh) respiration were highest with 4 g N m−2 yr−1, but autotrophic respiration (Ra) was highest with 8 to 16 g N m−2 yr−1. Also, the responses of Rh and Ra were unsynchronized considering the periods separately. N addition had no significant impact on the temperature sensitivity (Q10) for Rs but inhibited the Q10 for Rh. Significant interactions between observation period and N level occurred in soil respiration components and the temporal variations in soil respiration components were mostly associated with changes in microbial biomass carbon (MBC) and phospholipid fatty acids (PLFAs). Further observation on soil organic carbon and root biomass is needed to reveal the long-term effect of N deposition on soil C sequestration.
Similar content being viewed by others
Introduction
Nitrogen (N) limits terrestrial ecosystem productivity1. By the 2000s, China’s atmospheric N deposition had increased to 2 g N m−2 yr−1 2. The increase in N deposition concentrations greatly influences terrestrial ecosystem carbon (C) sequestration3. Reports on the effects of N addition on different components of soil respiration have been inconsistent. Soil autotrophic respiration (Ra) and heterotrophic respiration (Rh) have been found to be positively correlated with N addition with increased fine root biomass4,5, increased litter input6 and the priming effect resulting from the input of labile C substrates7, while researchers have also reported that soil respiration components are negatively correlated with N addition with reduced belowground C allocation, mycorrhizal hyphae4, microbial biomass8 and phenol oxidase concentration9.
Environmental conditions (soil texture, temperature and moisture) and organic carbon quality have been considered to be the primary factors for soil carbon mineralization models10,11. However, the biotic factors such as microbial community structure and activity are closely related to the soil carbon cycle; thus the contribution of soil microbes is gaining attention from researchers12. Rates of C sequestration in temperate grassland ecosystems range from 0 to >8 Mg C ha−1 yr−113. When studying the response of soil respiration to N addition in the grasslands of China, although plant variables (e.g., aboveground biomass, vegetation diversity and root: shoot ratio) have been commonly measured to reveal the mechanism by which N addition affects soil respiration4,14,15, soil microbial variables (e.g., microbial biomass, microbial community composition and the ratio among microbial groups) have been investigated only infrequently. In addition, previous studies have measured the respiration rate only between 08:00 and 11:00 hours during the day and the experimental N addition gradients were insufficient16,17; thus, actual or potential changes in soil respiration in response to N addition may have been misjudged.
In the present study, we measured soil respiration components at an experimental site that had been subjected to six levels of N addition for 10 yr. Soil physicochemical, vegetation and microbial variables were analyzed. Our objectives were to determine how the soil respiration components responded to N deposition during the growing season and how the environmental variables explained those responses. We hypothesized that (1) the responses of soil respiration components to N addition were nonlinear, (2) N addition increased the temperature sensitivity of soil respiration components and (3) the microbial variables played a substantial role in explaining the response of soil respiration components to the N addition.
Results
Respiration components
Averaged over all plots, the mean soil temperature was 15.6, 20.5 and 12.4 °C and the mean soil moisture was 10, 18 and 10% VWC (volumetric water content) in the early, middle and late observation periods, respectively. Although the mean soil temperature was lower in the early than in the middle observation period, the daily temperature variation was largest in the early observation period (Fig. 1). Daily soil moisture variation was relatively small, with ranges of only 2, 1 and 3% in the early, middle and late observation period, respectively (Fig. 1). The dynamics of soil respiration at six N addition levels followed similar temporal patterns, showing peak and valley occurrences. In the early and late growing season, respiration peaks and valleys occurred at 14:00–16:00 and 5:00–7:00; while in the middle growing season, respiration peaks and valleys occurred at 13:00–15:00 and 3:00–5:00.
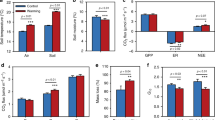
Respiration components significantly differed among the three observation periods (Table 1). The rates of total soil respiration (Rs), Rh and Ra were highest in the middle observation period and were lowest in the late observation period (Fig. 2). Averaged over all plots, Ra explained 40, 52 and 46% of Rs in the early, middle and late observation periods, respectively. The effects of N addition on respiration were nonlinear and differed among the respiration components (Table 1). Rs and Rh were significantly higher, with 4 g N m−2 yr−1, than with the other N levels, while Ra was highest, with 8 to 16 g N m−2 yr−1 (Fig. 2a,e,i). In the early observation period, Rs and Rh were generally higher with N addition than without N addition (Fig. 2b,f), while Ra was generally inhibited by N addition, except at 8 g N m−2 yr−1 (Fig. 2j). In the middle observation period, Rs insignificantly respond to N addition, except at 32 g N m−2 yr−1 (Fig. 2c); Rh was significantly lower with 2, 16 and 32 g N m−2 yr−1 than with 0 g N m−2 yr−1 (Fig. 2g), while Ra had no significant response to N addition (Fig. 2k). In the late observation period, Rs was slightly but significantly higher with 2 g N m−2 yr−1 than with the other five levels (Fig. 2d); Rh showed no consistent response to N addition (Fig. 2h), while Ra was generally stimulated by high levels of N addition (Fig. 2l).
Effects of N addition on soil respiration (a–d), heterotrophic respiration (e–h) and autotrophic respiration (i–l) averaged across the entire growing season and in the early, middle and late growing season, respectively. Rs is soil respiration, Rh is heterotrophic respiration, Ra is autotrophic respiration. Different letters indicate significant (P < 0.05) differences among N addition levels for each subplot, respectively. Errors bars represent standard error (n = 3 plot replicates).
Sensitivity of respiration to soil temperature and moisture
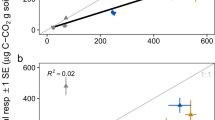
Soil temperature had significantly positive correlations with Rs and Rh on a daily scale. The Q10 values for Rs ranged between 1.15 and 1.55 and the Q10 values for Rh ranged between 1.28 and 2.41. Because Ra was not sensitive to the daily dynamics of the soil temperature in this study, the Q10 for Ra was not calculated. Although the respiration components were not correlated with soil moisture on a daily scale, soil moisture explained 72, 91 and 44% of the variation in Rs, Rh and Ra across the entire growing season if all N addition levels were considered. Therefore, in addition to being regulated by soil temperature, respiration was also regulated by soil moisture. The Q10 for Rs increased with soil moisture (Fig. 3a) but was not significantly affected by the level of N addition (Fig. 3b). The Q10 for Rh was unrelated to soil moisture (Fig. 3c) but decreased as N addition increased (Fig. 3d).
The effects of N addition on soil properties and microorganisms
Comparing the pre-growing and growing season, soil total organic carbon (TOC) and total nitrogen (TN) did not change significantly according to paired-sample t-tests. Total phosphorus (TP) was negatively correlated with the N addition level both in the pre-growing and growing season, while TOC, TN and TOC: TN (C: N) ratio showed no significant pattern in response to the N addition level (Table 2, Supplementary information Table S3). In August, plant aboveground biomass was positively correlated with the N level, but plant species richness was negatively correlated with the N level (Table 2).
Across the entire growing season, soil inorganic nitrogen (NH4+ -N and NO3−-N) and dissolved organic carbon (DOC) were positively correlated with the N level, while pH value and microbial biomass carbon (MBC) were negatively correlated with the N level (Table 2). The highest inorganic N content in the middle growing season indicated urea hydrolysis occurred after N addition, but the difference of pH between the early and middle growing season was insignificant (P = 0.167) according to the paired-sample t-tests, suggesting the change of pH caused by urea hydrolysis were ignorable (Supplementary information Figure S2 and S3). DOC was highest in the middle growing season, while MBC peaked in the late observation period.
Although the abundance of soil phospholipid fatty acids (PLFAs) and the fungi: bacteria (F: B) ratio were not correlated with the N addition level when data were pooled across the entire growing season, the microbial community composition was changed by N addition in different observation periods. In the middle observation period, negative correlations occurred between microbial community PLFAs and N levels (Table 2). With increases in the N level, the monoenoic: saturated PLFA ratio decreased in the middle observation period and the F: B ratio decreased in the late observation period (Table 2).
The relationship between environmental variables and respiration
In a PCA based on 54 samples (18 plots × three observation periods), the first axis explained 77.8% of the variance in the respiration data, whereas the second axis explained 20.0% (Fig. 4). The PCA showed a clear separation of plots over the early, middle and late growing seasons.
Principal component analysis (PCA) of soil respiration components in the growing season.
Circles, upward triangles, boxes, diamonds, downward triangles and squares represent respiration with 0, 2, 4, 8, 16 and 32 g N m−2 yr−1 plot, respectively. Black, grey and white symbols represent respiration in the early, middle and late growing season, respectively.
Using the interaction between observation period and environmental variables, we examined how the interaction changed relative to the average change in soil respiration components during the growing season. The first two axes of the RDA explained 68.7% of the variance in the relationship between soil respiration components and environmental variables (Fig. 5). Soil respiration components were positively correlated with PLFAs, while respiration was negatively correlated with MBC (Fig. 5).The effect of each environmental variable on the soil respiration components was identified. Among the variables, PLFAs and MBC explained most of the temporal changes in the soil respiration components (Table 3).
Redundancy analysis (RDA) of soil respiration components in the growing season.
MBC is microbial biomass carbon, DOC is dissolved organic carbon, NH4+-N is ammonium nitrogen, NO3−-N is nitrate nitrogen, mono: sat is monoenoic: saturated PLFAs ratio, F: B is fungi: bacteria ratio, pH is soil pH value, op is observation period.
Discussion
Averaged across the entire growing season, the threshold was 4 g N m−2 yr−1 for Rh, but 8–16 g N m−2 yr−1 for Ra. Considering the three observation periods separately, the responses of Rs and Ra to N addition were unsynchronized in each period. The increase in Rs in the early observation period in response to N addition primarily resulted from an increase in Rh rather than Ra. This result is reasonable because soil microorganisms become active earlier in the growing season than do plants and accumulation of DOC from the previous year can stimulate Rh. The correlation between DOC and N level in the early growing season most likely resulted from an increase in litter input or microbial cell lysis18,19. Inorganic N content was highest after N addition in the middle observation period, a period when Rh was substantially inhibited by N addition. Research has demonstrated that N fertilizer reduces the synthesis of various energy-consuming oxidative enzymes, such as phenol oxidase and peroxidase9,20, leading to a limitation of substrate resources and a decrease in soil respiration. Hence, there may exist one phenomenon of starvation-survival, which is defined as a physiological state resulting from the insufficient energy (catabolism) and nutrients (anabolism) availability for microbial growth and reproduction21,22. Microbial starvation was induced by N addition in the middle growing season, indicated by the negative correlation between MBC and N level, the failure of DOC to increase with N addition and the decrease in the ratio of monoenoic: saturated PLFAs with N addition. The promotion of Ra by N addition was reported to be caused by increased root biomass23. However, previous research at this study site had concluded that root biomass did not increase with N addition24. N deposition can switch C4 plants to C3 plants25 and induce the replacement of K-strategy species (perennial grasses and forbs) by r-strategy species (early successional annuals)26. Decreases in plant species richness as a result of N addition have been shown in this study. We suspect that no significant response of Ra to N addition resulted from the changes in root physiology as the plant community changed27. Late in the growing season, Rs was insignificantly different among N addition conditions except for 2 g N m−2 yr−1, due to the balance between Ra and Rh with 4 to 32 g N m−2 yr−1. The decrease in the F: B ratio suggests that the N addition dampens the fungi more than bacteria, thus making the microbial community prefer to use the relatively labile soil organic matter28,29.
The Q10 value for Rs and Rh presented different responses to soil moisture and N addition. The Q10 value for Rs at our study site ranged from 1.15 to 1.55, which is consistent with a previous study conducted in Inner Mongolia, China30. The positive correlation between soil moisture and the Q10 value for Rs is consistent with previous reports31,32. However, in contrast to the findings from a previously published incubation experiment conducted at this study site33, the Q10 value for Rh showed no clear response to soil moisture, suggesting that N addition may interfere the microbial response of Q10 to soil moisture. Our original hypothesis is that the N addition promotes the temperature sensitivity of soil respiration because the N limitation could be relieved for plant and microbes34,35. Surprisingly, N addition showed no clear effect on the Q10 value for Rs and the Q10 value for Rh was significantly reduced by N addition in this study. Mo et al.36 considered that the differed response of Rs Q10 values to N addition mainly result from the variance in soil nutrient content among the study sites. In addition, Microbial groups have been reported to have different preferences for soil temperature, moisture and substrate7,37. The Q10 value for Rh was reported to be higher in soils with high F: B ratios in an incubation experiment38. Therefore, we suspect that the low content of TOC in the study site and varying levels of tolerance to N addition among the microbial community might explain the response of the Q10 value for Rs and Rh to N addition.
Peaked in the middle growing season, Ra had a significantly positive correlation with daily mean soil temperature considering the whole growing season (R = 0.876, P < 0.001). However, due to its complex structure and adaptation mechanisms involved, plant might be more homeostatic than microbes to short-term environmental change, such as the 24 h dynamics of soil temperature in this study. Besides, the suppression in mycorrhizal respiration may also inhibit the sensitivity of Ra to the daily changes in soil temperature, since the arbuscular mycorrhizal fungi (AMF) were found significantly reduced by N addition in the middle observation period, consistent with previous studies39.
The N thresholds were found to differ among the microbial variables and plant variables in this study. MBC was low throughout the growing season in plots treated with 8–32 g N m−2 yr−1, which is consistent with the finding that the critical level of N deposition for MBC is <5 g N m−2 yr−1 for typical temperate steppes in China40. Moreover, nonlinear responses to N addition have also been reported for MBC and plant biomass in other experiments26,29. In contrast, the 32 g N m−2 yr−1 did not seem to be a saturation point for the response of aboveground biomass, although plant species richness was inhibited by N addition.
The interactive effects of environmental variables and observation periods were considered on soil respiration components. Time is treated as a potential regulator because it can gradually modify the environmental variables at long term scale. In other words, soil respiration is regulated by the legacy effect of environmental variables subjected to N addition. MBC and PLFAs explained most of the temporal changes in the soil respiration components in the current study. Therefore, we recommend that soil microbial variables (e.g., microbial biomass, microbial community composition and the ratio among microbial groups) be more commonly investigated to reveal the mechanism by which N addition affects soil respiration.
Variation occurs in the responses of belowground carbon compartments to N addition. The correlation between N level and DOC changed through the growing season, while TOC was not correlated with N level, the increase in DOC did not contribute to changes in TOC because DOC is low in the study site and is generally suggested to have minor contribution for C sequestration41. TOC stays relatively stable between the pre- and middle growing season and one study in 2011 concluded that root biomass had no significant response to N addition at this study site24. It may be too hasty to draw the conclusion that nitrogen has no significant impact on soil C after 10-year N addition, because we only studied soil nutrient contents for one growing season. In previous studies at the same study site, we also found soil organic matter had no significant difference among N levels after 3-year and 8-year N addition, respectively24,42. Although the effects of N addition on soil C storage might differ across studies, possibly due to site variation in soil texture43, the lack of response of soil C to N addition also occurred in other grassland ecosystems44,45. It is well acknowledged that N deposition substantially influences terrestrial ecosystem C sequestration, but a point of view is raised that N addition enhances aboveground C rather than soil C46. Therefore, information on soil organic carbon and root biomass need to be consistently traced, in order to reveal the effect of N on soil C sequestration at long term scale.
Soil pH value should be taken into consideration in N deposition experiments, although soil pH has no significant impact on the temporal variability of soil respiration components in this study. Previous study has concluded that two potential regulators by N addition should be addressed: N availability and soil acidification41, it is possible that soil acidification indirectly impacts soil respiration by shifting the plant community to species with higher acid tolerance and higher specific root respiration47,48, suppressing soil microbial biomass, enzymatic activity49,50 and changing the microbial structure to lower F: B ratio48,51.
Plot trenching is commonly used to estimate Rh and Ra because of its simplicity and low cost. However, there are inaccuracies in the methodology of trenching that need to be considered. Although we endeavored to minimize the effect, soil disturbance would accelerate the decomposition of soil organic matter which exposed to the air. Soil microbial structure and function will be affected when the plant-derived carbon (e.g., plant residues and rhizodeposition), which stimulates the priming effect of soil organic matter52,53, changes in the trenched plot. In addition to substrate availability, changes in soil temperature and moisture caused by trenching also regulate soil microbial activity54. In fact, roots can both affect Ra and Rh in the soil, for the rhizosphere-derived CO2 results from root respiration, rhizo-microbial respiration, microbial respiration of dead plant residues and additional SOM-derived CO2 via the priming effect55,56. Grass root severing in the trenched plots can result in an inaccuracy in measuring Rh because substrate quality is changed by increased dead root decomposition occurring with decreased photosynthates from aboveground56. Normally, a lack of photosynthates would be significant only after a long period of stabilization for a trenched plot56. Trenching would lead to the overestimation of Rh in which the CO2 derived from the extra root decomposition was included.
Methods
Study area
The study area is located in Duolun County, Inner Mongolia, China (42.02°N, 116.17°E; 1,341 m a.s.l.). The annual mean temperature is 2.1°C and the mean annual precipitation is 385.5 mm; the typical soil in this region is chestnut, composed of 62.8% sand, 20.3% silt and 17.0% clay, the bulk density is 1.31 g cm−3 and the dominant vegetation species are Stipa krylovii, Leymus chinensis, Artemisia frigida, Agropyron cristatum and Allium bidentatum24. The growing season in the study site is proximately from May to September. Phenophase of budding mainly occurs from April to June; inflorescence, tasseling and flowering occurs from July to August; And fruiting to senescing occurs from September to October57.
The N addition experiment included six N levels (0, 2, 4, 8, 16 and 32 g N m−2 yr−1) and three replicates for each level. The replicates were assigned to 18 plots (15 × 10 m, separated from each other by a 4-m-wide buffer zone) following a Latin square design (Supplementary Figure S1). In mid-July (during the rainy season) of each year, referred to the weather forecast, dry urea (CO(NH2)2) has been manually spread on the surface of the plots before the rain since July 2003.
Respiration measurement
Soil heterotrophic respiration (Rh, μmol of CO2 m−2 s−1) was measured using the trenching method. On 23 April, 2013, one 40 cm × 40 cm subplot was formed in each plot by inserting iron plates 30 cm deep into the soil. The aboveground vegetation in the subplots was clipped and the plant tillers and roots in the top 0–10 cm of the soil were carefully removed. In order to maximize the soil integrality in the subplots, instead of sieving roots out of the whole soil block, we only yanked out the clods in the top 0–10 cm of the soil where plant tillers were situated (the magnitude of the clod depended on the tiller). The plant tillers and the roots in the clods were removed before the rootless soil was subsequently refilled and the remaining roots in the subplots were left to be decomposed. Weeds above the soil in the subplots were clipped if they existed once every 10 days. Twenty-four h before the observation on 22 May, a PVC collar (20 cm diameter, 14 cm height) was inserted in the middle of each subplot to measure Rh. A second PVC collar was inserted at a randomly selected location outside of the subplot but inside the plot, with aboveground removed and belowground vegetation retained, to measure soil total respiration (Rs, μmol of CO2 m−2 s−1). All collars were inserted 6–7 cm deep and left in place after they were inserted into the soil.
Respiration was measured during approximately 14-day periods in the early growing period (from 22 May to 4 June), middle growing period (from 23 July to 4 August) and late growing period (from 20 September to 4 October) in 2013, respectively. During each period, 18 plots were each observed once. The respiration rates in three plots with six collars were measured on each date and the measurement would be postponed for 1–2 days after precipitation to avoid CO2 flux pulse. Respiration rate was measured with a soil C-flux automatic measurement system (LI-COR, NE, USA). Meanwhile, soil temperature (°C) and moisture (volumetric water content, %VWC) in the top 0–10 cm layer of the soil near the collar were measured with an auxiliary sensor attached to the LI-COR 8150 and data for each collar were recorded once every 1 h to track the daily dynamics.
One thing to note here is that soil respiration measured between 08:00 and 11:00 was not suitable to represent the mean respiration for 24 h in this study. We treated the mean respiration for 08:00–11:00 as the univariate predictor to fit the mean respiration for 24 h, with an intercept of zero. The R2 values indicated that except for the early growing season (y = 1.002×, R2 = 0.79), soil respiration measured between 08:00 and 11:00 failed to represent the mean respiration for 24 h (the middle growing season: y = 0.975×, R2 = 0.47; the late growing season y = 1.0469 x, R2 = 0.46).
Sampling and analysis
In April, May, July and September of 2013, we randomly collected three soil samples (0–20 cm depth) in each plot (outside the subplot); these were combined to form one composite sample per plot. Air-dried soil samples were grind and sieved through 0.25 mm before analysis of total organic carbon (TOC, g kg−1), total phosphorus (TP, g kg−1) and total nitrogen (TN, g kg−1). TOC was measured by the potassium dichromate-oxidation method, TP was measured with a spectrometer (SPECTRO, Kleve, Germany) and TN was measured with an elemental analyzer (Perkin-Elmer, MA, USA).
Fresh soil samples were sieved through 2 mm and prepared to measure soil pH, soil inorganic nitrogen (NH4+-N and NO3−-N, mg kg−1), microbial biomass carbon (MBC, mg kg−1), dissolved organic carbon (DOC, mg kg−1) and soil phospholipid fatty acids (PLFAs, μmol g−1). Soil pH was measured in a 1: 2.5 (soil: water) suspension. Deionized water was added to the soil and soil solution was extracted from the soil samples after vortex and centrifuge. Ammonium nitrogen (NH4+-N) and nitrate nitrogen (NO3−-N) were measured from the solution using ion chromatograph analyzers (DIONEX, CA, USA).
MBC was determined by the chloroform fumigation-extraction method58. Soil samples were divided into the fumigated group (chloroform fumigated for 24 h) and the control group. 0.5 M K2SO4 solution was used to extract soil organic carbon from both groups. The extracts were measured with a TOC analyzer (Elementar, Hanau, Germany). MBC was determined by multiplying the difference of extracted organic carbon between fumigated and control soil by 0.45, the conversion factor. DOC was determined as the extracted organic carbon in the control soil.
PLFAs were measured referring to Bligh & Dyer59. The extracts were measured with gas chromatography/mass spectrometry (Agilent Technologies, CA, USA). Based on previous research28,29,39, the groups of microorganisms were divided into bacteria (Gram-positive bacteria and Gram-negative bacteria), fungi, actinomycetes and arbuscular mycorrhizal fungi (AMF). Decreased ratio of monoenoic to saturated fatty acids is a useful stress signature to provide the profiles of the starving status of natural microbial communities60, thus the ratio of monoenoic: saturated PLFAs was measured in this study (Supplementary Table S1).
In early August of 2013, aboveground plant biomass was removed by clipping in a 1 m × 1 m area randomly located area in each plot (outside of the subplot). The clippings were oven dried at 65°C to a constant weight to assess aboveground plant biomass (g m−2). Before drying, the clippings were examined to determine species richness.
Statistical analysis
Equation (1) was used to estimate Rh from the plot used to measure Rs in the growing season:

where Rh is the actual heterotrophic respiration rate (μmol CO2 m−2 s−1), T is the soil temperature (°C, near the collar used to measure Rh) and M is the soil moisture (%VWC, near the collar used to measure Rh). Parameters a through d were estimated and the resulting equations adequately described the data (Supplementary Table S2). Therefore, the estimated heterotrophic respiration rate is obtained by substituting parameters of a through d, soil temperature and moisture near the collar (for measuring Rs) into equation (1). In addition, soil autotrophic respiration (Ra, μmol CO2 m−2 s−1) is the difference of Rs and estimated Rh.
To evaluate the response of the respiration components to temperature, Q10 was measured by the following exponential functions:


where R is either Rs, estimated Rh or Ra(μmol CO2 m−2 s−1) and T is the soil temperature (°C) near the collar used to measure Rs. Parameter a indicates the basal respiration rate and b is the exponent used to calculate Q10.
A repeated-measures ANOVA was used to assess how the interaction between observation period and the N addition level affected the respiration components. A one-way ANOVA was used to examine the effect of the N addition level on the respiration components, MBC, DOC, inorganic N (NH4+ and NO3-) and microbial PLFAs. Paired-sample t-tests were used to determine whether the soil properties (TOC, TN, TP and pH) differed between the sampling periods. A Pearson correlation analysis was also used to determine the relationship between N level, inorganic nitrogen, MBC, DOC, PLFAs and aboveground biomass. The statistical analyses were conducted using IBM SPSS 20.0 (IBM, NY, USA). Graphs were created using Sigmaplot 12.5 (Systat Software, CA, USA).
A principal component analysis (PCA) and a redundancy analysis (RDA) were conducted using CANOCO 4.5 (Microcomputer Power, NY, USA) to determine which aggregates of environmental variables best explained the variances of soil respiration components. Data for three respiration components (Rs, Rh and Ra) and 54 plots (6 N levels × 3 replicates × 3 observation periods) were used to describe the variances in soil respiration components as affected by N level and observation period and a split-plot design was used to avoid the autocorrelation between the individual observations in both the respiration and the environmental variables. Monte Carlo tests were used in the automatic forward selection procedure to identify the interactive effects of environmental variable and observation period on soil respiration components.
Additional Information
How to cite this article: Li, Y. et al. Microbial properties explain temporal variation in soil respiration in a grassland subjected to nitrogen addition. Sci. Rep. 5, 18496; doi: 10.1038/srep18496 (2015).
References
Treseder, K. K. Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11, 1111–1120 (2008).
Liu, X. J. et al. Enhanced nitrogen deposition over China. Nature 494, 459–462 (2013).
Gruber, N. & Galloway, J. N. An Earth-system perspective of the global nitrogen cycle. Nature 451, 293–296 (2008).
Hasselquist, N. J., Metcalfe, D. B. & Hogberg, P. Contrasting effects of low and high nitrogen additions on soil CO2 flux components and ectomycorrhizal fungal sporocarp production in a boreal forest. Global Change Biol 18, 3596–3605 (2012).
Zhou, L. Y. et al. Different responses of soil respiration and its components to nitrogen addition among biomes: a meta-analysis. Global Change Biol 20, 2332–2343 (2014).
Gentilesca, T., Vieno, M., Perks, M. P., Borghetti, M. & Mencuccini, M. Effects of Long-Term Nitrogen Addition and Atmospheric Nitrogen Deposition on Carbon Accumulation in Picea sitchensis Plantations. Ecosystems 16, 1310–1324 (2013).
Bird, J. A., Herman, D. J. & Firestone, M. K. Rhizosphere priming of soil organic matter by bacterial groups in a grassland soil. Soil Biol Biochem 43, 718–725 (2011).
Jiang, X. Y., Cao, L. X. & Zhang, R. D. Changes of labile and recalcitrant carbon pools under nitrogen addition in a city lawn soil. J Soil Sediment 14, 515–524 (2014).
Zak, D. R., Pregitzer, K. S., Burton, A. J., Edwards, I. P. & Kellner, H. Microbial responses to a changing environment: implications for the future functioning of terrestrial ecosystems. Fungal Ecol 4, 386–395 (2011).
Parton, W. J. & Singh, J. S. Adapting a biomass simulation-model to a tropical grassland. Ecol Model 23, 151–163 (1984).
Kemp, P. R., Reynolds, J. F., Virginia, R. A. & Whitford, W. G. Decomposition of leaf and root litter of Chihuahuan desert shrubs: effects of three years of summer drought. J Arid Environ 53, 21–39 (2003).
Garcia-Pausas, J. & Paterson, E. Microbial community abundance and structure are determinants of soil organic matter mineralisation in the presence of labile carbon. Soil Biol Biochem 43, 1705–1713 (2011).
Jones, M. B. & Donnelly, A. Carbon sequestration in temperate grassland ecosystems and the influence of management, climate and elevated CO(2). New Phytol 164, 423–439 (2004).
Oishi, A. C., Palmroth, S., Johnsen, K. H., Mccarthy, H. R. & Oren, R. Sustained effects of atmospheric [CO2] and nitrogen availability on forest soil CO2 efflux. Global Change Biol 20, 1146–1160 (2014).
Rodriguez, A. et al. Lability of C in temperate forest soils: Assessing the role of nitrogen addition and tree species composition. Soil Biol Biochem 77, 129–140 (2014).
Xu, W. H. & Wan, S. Q. Water- and plant-mediated responses of soil respiration to topography, fire and nitrogen fertilization in a semiarid grassland in northern China. Soil Biol Biochem 40, 679–687 (2008).
Yan, L. M., Chen, S. P., Huang, J. H. & Lin, G. H. Differential responses of auto- and heterotrophic soil respiration to water and nitrogen addition in a semiarid temperate steppe. Global Change Biol 16, 2345–2357 (2010).
Breulmann, M., Schulz, E., Huhn, K. W. & Buscot, F. O. Impact of the plant community composition on labile soil organic carbon, soil microbial activity and community structure in semi-natural grassland ecosystems of different productivity. Plant Soil 352, 253–265 (2012).
Henry, H. A. L. & Moise, E. R. D. Grass litter responses to warming and N addition: temporal variation in the contributions of litter quality and environmental effects to decomposition. Plant Soil 389, 35–43 (2015).
Cusack, D. F., Silver, W. L., Torn, M. S., Burton, S. D. & Firestone, M. K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 92, 621–632 (2011).
Lever, M. A. et al. Life under extreme energy limitation: a synthesis of laboratory- and field-based investigations. Fems Microbiol Rev 39, 688–728 (2015).
Mortia, R. Y. The starvation-survival state of microorganisms in nature and its relationship to the bioavailable energy. Experientia 46, 813–817 (1990).
Zhang, C. P. et al. Effects of simulated nitrogen deposition on soil respiration components and their temperature sensitivities in a semiarid grassland. Soil Biol Biochem 75, 113–123 (2014).
Song, B., Niu, S. L., Li, L. H., Zhang, L. X. & Yu, G. R. Soil carbon fractions in grasslands respond differently to various levels of nitrogen enrichments. Plant Soil 384, 401–412 (2014).
Zeng, D. H. et al. Effects of nitrogen addition on vegetation and ecosystem carbon in a semi-arid grassland. Biogeochemistry 98, 185–193 (2010).
Bai, Y. F. et al. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from inner Mongolia Grasslands. Global Change Biol 16, 358–372 (2010).
Laganiere, J., Pare, D., Bergeron, Y. & Chen, H. The effect of boreal forest composition on soil respiration is mediated through variations in soil temperature and C quality. Soil Biol Biochem 53, 18–27 (2012).
Feng, X. J. & Simpson, M. J. Temperature and substrate controls on microbial phospholipid fatty acid composition during incubation of grassland soils contrasting in organic matter quality. Soil Biol Biochem 41, 804–812 (2009).
Wei, C. Z. et al. Nitrogen deposition weakens plant-microbe interactions in grassland ecosystems. Global Change Biol 19, 3688–3697 (2013).
Chen, Q. S. et al. Responses of soil respiration to temperature in eleven communities in Xilingol Grassland, Inner Mongolia. Acta Phytoecologica Sinica 27, 441–447 (2003).
Rey, A., Petsikos, C., Jarvis, P. G. & Grace, J. Effect of temperature and moisture on rates of carbon mineralization in a Mediterranean oak forest soil under controlled and field conditions. Eur J Soil Sci 56, 589–599 (2005).
Yuste, C. J. et al. Microbial soil respiration and its dependency on carbon inputs, soil temperature and moisture. Global Change Biol 13, 2018–2035 (2007).
Li, Y., Liu, Y. H., Shen, W. J., Xu, X. & Tian, Y. Q. Responses of soil heterotrophic respiration to changes in soil temperature and moisture in a Stipa krylovii grassland in Nei Mongol. Acta Phytoecologica Sinica 38, 238–248 (2014).
Lagomarsino, A., De Angelis, P., Moscatelli, M. C. & Grego, S. The influence of temperature and labile C substrates on heterotrophic respiration in response to elevated CO2 and nitrogen fertilization. Plant Soil 317, 223–234 (2009).
Coucheney, E., Stromgren, M., Lerch, T. Z. & Herrmann, A. M. Long-term fertilization of a boreal Norway spruce forest increases the temperature sensitivity of soil organic carbon mineralization. Ecol Evol 3, 5177–5188 (2013).
Mo, J. M. et al. Response of soil respiration to simulated N deposition in a disturbed and a rehabilitated tropical forest in southern China. Plant Soil 296, 125–135 (2007).
Drenovsky, R. E., Steenwerth, K. L., Jackson, L. E. & Scow, K. M. Land use and climatic factors structure regional patterns in soil microbial communities. Global Ecol Biogeogr 19, 27–39 (2010).
Zhou, W. P., Hui, D. F. & Shen, W. J. Effects of Soil Moisture on the Temperature Sensitivity of Soil Heterotrophic Respiration: A Laboratory Incubation Study. PLOS ONE 9, 10.1371/journal.pone.0092531 (2014).
van Diepen, L. T. A., Lilleskov, E. A., Pregitzer, K. S. & Miller, R. M. Simulated Nitrogen Deposition Causes a Decline of Intra- and Extraradical Abundance of Arbuscular Mycorrhizal Fungi and Changes in Microbial Community Structure in Northern Hardwood Forests. Ecosystems 13, 683–695 (2010).
Liu, X. J. et al. Nitrogen deposition and its ecological impact in China: An overview. Environ Pollut 159, 2251–2264 (2011).
Chen, H. et al. Effects of nitrogen deposition on carbon cycle in terrestrial ecosystems of China: A meta-analysis. Environ Pollut 206, 352–360 (2015).
Zhang, N. et al. Impacts of urea N addition on soil microbial community in a semi-arid temperate steppe in northern China. Plant Soil 311, 19–28 (2008).
Riggs, C. E., Hobbie, S. E., Bach, E. M., Hofmockel, K. S. & Kazanski, C. E. Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry 125, 203–219 (2015).
Zeng, D. H. et al. Effects of nitrogen addition on vegetation and ecosystem carbon in a semi-arid grassland. Biochemistry 98, 185–193 (2010).
Skinner, R. H. Nitrogen fertilization effects on pasture photosynthesis, respiration and ecosystem carbon content. Agr Ecosyst Environ 172, 35–41 (2013).
Lu, M. et al. Minor stimulation of soil carbon storage by nitrogen addition: A meta-analysis. Agr Ecosyst Environ 140, 234–244 (2011).
Chen, D., Li, J., Lan, Z., Hu, S. & Bai, Y. Soil acidification exerts a greater control on soil respiration than soil nitrogen availability in grasslands subjected to long-term nitrogen enrichment. Funct Ecol 10.1111/1365-2435.12525 (2015).
Chen, D. et al. Biotic community shifts explain the contrasting responses of microbial and root respiration to experimental soil acidification. Soil Biol Biochem 90, 139–147 (2015).
Wang, R. Z. et al. Coupled response of soil carbon and nitrogen pools and enzyme activities to nitrogen and water addition in a semi-arid grassland of Inner Mongolia. Plant Soil 381, 323–336 (2014).
Geisseler, D., Horwath, W. R. & Doane, T. A. Significance of organic nitrogen uptake from plant residues by soil microorganisms as affected by carbon and nitrogen availability. Soil Biol Biochem 41, 1281–1288 (2009).
Wei, C. et al. Nitrogen deposition weakens plant-microbe interactions in grassland ecosystems. Global Change Biol 19, 3688–3697 (2013).
Bradford, M. A., Keiser, A. D., Davies, C. A., Mersmann, C. A. & Strickland, M. S. Empirical evidence that soil carbon formation from plant inputs is positively related to microbial growth. Biogeochemistry 113, 271–281 (2013).
Blagodatskaya, E. et al. Microbial interactions affect sources of priming induced by cellulose. Soil Biol Biochem 74, 39–49 (2014).
Chen, D. M. et al. Subtropical plantations are large carbon sinks: Evidence from two monoculture plantations in South China. Agr Forest Meterol 151, 1214–1225 (2011).
Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol Biochem 38, 425–448 (2006).
Subke, J., Inglima, I. & Francesca Cotrufo, M. Trends and methodological impacts in soil CO2 efflux partitioning: A metaanalytical review. Global Change Biol 12, 921–943 (2006).
Zhang, F., Zhou, G. S. & Wang, Y. H. Pheonological calendar of Stipa Krylovii steppe in Inner Mongolia, China and its correlation with climatic variables. J Plant Ecol (Chinese Version) 32, 1312–1322 (2008).
Vance, E. D., Brookes, P. C. & Jenkinson, D. S. An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19, 703–707 (1987).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Canadian J Physio Biochem 37, 911–917 (1959).
Kieft, T. L., Ringelberg, D. B. & White, D. C. Changes in ester-linked phospholipid fatty-acid profiles of subsurface bacteria during starvation and desiccation in a porous-medium. Appl Environ Microb 60, 3292–3299 (1994)
Acknowledgements
This work was funded by the National Natural Science Foundation of China (31270500, 31240002), the CAS Strategic Priority Research Program (XDA05050602) and the Fundamental Research Funds for the Central Universities (Grant No. 2014KJJCB01). We thank the Duolun Restoration Ecology Research Station (part of the Institute of Botany, Chinese Academy of Sciences) for providing access to the study site.
Author information
Authors and Affiliations
Contributions
Y.H.L. designed the study, proposed the scientific hypothesis and supervised the project; Y.L., S.M.W. and L.N. collected field samples and data; Y.L. analyzed the data and prepared the figures; Y.L. and Y.H.L. wrote the manuscript; Y.H.L. and Y.Q.T. provided editorial advice. All authors discussed the results and reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, Y., Liu, Y., Wu, S. et al. Microbial properties explain temporal variation in soil respiration in a grassland subjected to nitrogen addition. Sci Rep 5, 18496 (2016). https://doi.org/10.1038/srep18496
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep18496
This article is cited by
-
Drivers of soil respiration across a management intensity gradient in temperate grasslands under drought
Nutrient Cycling in Agroecosystems (2022)
-
Soil respiration and N-mineralization processes in the Patagonian steppe are more responsive to fertilization than to experimental precipitation increase
Plant and Soil (2022)
-
Variations in the nitrogen saturation threshold of soil respiration in grassland ecosystems
Biogeochemistry (2020)
-
Responses of soil microbes and their interactions with plant community after nitrogen and phosphorus addition in a Tibetan alpine steppe
Journal of Soils and Sediments (2020)
-
Urea fertilization decreases soil bacterial diversity, but improves microbial biomass, respiration, and N-cycling potential in a semiarid grassland
Biology and Fertility of Soils (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.